Meningiomas are most prevalent primary intracranial tumour and spinal intradural neoplasms [1], presenting over one third of all Central Nervous System (CNS) tumours in adults, with the ratio between female: male is 2:1. Imaging studies and autopsy indicate that the incidence of subclinical meningiomas is nearly 3% in the population [2].
Histological grading is considered the most important factor in meningioma prognosis [3]. However, after complete surgical resection of meningiomas, and in spite of bone and dura mater involvement, recurrence occurs in 80% of incomplete surgical resection and 20% of cases with complete surgical resection even in some low grade tumours cases [4]. This has led to study of other factors in order to predict the behavior of meningiomas as use of different immunohistochemical markers for detecting meningioma progression [5].
Among the cell adhesion molecules, CD44 is assumed to be related to tumour invasion and metastatic ability. It was studied on several tumours types, including meningiomas [6]. CD44 is a cell surface glycoprotein receptor which is ligand to hyaluronan; an extracellular matrix molecule, being implicated in matrix mediated cell signaling and in cell-matrix adhesion. It is expressed on almost all human cells. It is important for regulating of cell adhesion, migration, angiogenesis, proliferation and inflammation [7-9].
The studies showed that the expression of anti CD44 antibodies; using immunohistochemical technique in meningiothelial, transitional subtypes and higher grade tumours, was more intense than lower grade of tumours where the expression in such types was highly variable [10-14]. Additionally, the relationship between brain-invasive growth and CD44 expression in meningioma has been addressed by few studies without finding a clear association [10,14].
Also, among these immunohistochemical markers is identification of markers which are responsible for the controlling the cell proliferation. Ki-67 antigen is a non-histone protein marker that is only expressed in the active proliferative phase of the cell cycle, and can be simply examined on formalin fixed paraffin embedded tissue sections. Proliferation index estimated by Ki-67 may be used as a way for expecting meningioma behavior [15-19].
The aim of this study was to evaluate the immunohistochemical expression of CD44 and Ki-67 in different histological types of meningioma and correlate the results of their expression with various clinicopathologic variables.
Materials and Methods
Study Group
This cross-sectional study was performed at the Pathology department, Kasr Al Ainy Hospital, Faculty of Medicine, Cairo University from January 2015 to July 2016. It included 40 cases of intracranial meningioma.
The study was conducted in correspondence with the guidelines of the local ethical committee of the faculty of medicine, Cairo University and took the approval of ethical committee.
Cases with deficient clinical data or poorly fixed specimens were not included in the study.
Histopathological Evaluation
Formalin fixed and paraffin embedded blocks were sectioned at 5 µm thickness and examined microscopically using H&E stain to evaluate the histological diagnosis which was performed according to the criteria of the WHO 2016.
Immunohistochemical Procedure of CD44 and Ki-67
The archived 40 paraffin embedded blocks of the meningioma cases were sectioned on adhesive charged microscopic slides, 5 µ sections were obtained. According to Dako standard protocol heat mediated antigen retrieval was performed with citrate buffer pH 6 in automated water bath (Dako PT link, PT101). The primary antibodies were CD44 Std. /HCAM Ab-4 (#MS-668-R7), (7.0ml), manufactured by: NeoMarkers For Lab Vision Corporation, UK, against CD44 protein and Ki-67, monoclonal antibody, 1:50, DAKO, USA. Immunohistochemical staining was performed in an autostainer (Dako autostainer link 48) using a polymer-based detection system (DakoEnVision™ FLEX, K8000). Diaminobenzidine (DAB) was used as chromogen and Mayer’s hematoxylin was used as a counter stain, then after the cover slips and DPX mounting medium were used for mounting and preserving sections. All sections were examined by using Olympus BX51 light microscope with 20X and 40X objective eye pieces. The positive control used for CD44 and Ki-67 were tonsillar tissue and a case of invasive duct carcinoma with high Ki-67 proliferation index respectively.
Evaluation of Expression of both CD44 and Ki-67
Immunostaining of CD44 was evaluated semi quantitatively for the presence of membranous or cytoplasmic staining and graded as mild, moderate and marked when the percentage of CD44 positive staining cells was as follows: 0-5%; from 5 to 50% and > 50% respectively [20]. Ki-67 expression was determined as percentage through counting in 100 nuclei in the busiest foci of the tumour.
Cells in areas with necrosis, poor morphology or at the margins of sections were not counted in both markers.
The results of CD44 and Ki-67 immunostaining were correlated with multiple prognostic factors (age, sex, histopathological type, histopathological grade, brain invasion and recurrence).
Statistical Analysis
Statistical Package for Social Sciences (SPSS) version 22.0 was used for statistical analysis. Numerical data were summarized using means and standard deviations. Categorical data were summarized as percentages. Data were explored for normality using Kolmogorov-Smirnov test and Shapiro-Wilk test. Comparisons between the two groups were done using the t-test. For categorical variables, differences were analysed with Chi-square (χ2) test and Fisher’s-exact test when appropriate. Pearson correlation coefficient was used to assess correlation between numeric variables. The p-values ≤0.05 were considered significant.
Results
The patients’ age ranged from 12 to 75 years with mean age 49.5±14.89 years. Twenty seven patients were females while 13 were males.
CD44 cytoplasmic and membranous immunostaining was graded as mild in 21 cases (52.5%), moderate in 8 cases (20%) and marked in 11 cases (27.5%). Correlation between CD44 expression and clinicopathologic variables is summarized in [Table/Fig-1]. A significant positive correlation was detected between CD44 expression and meningioma grade as marked CD44 expression was detected in 9 cases (81.8%) of high grades II and III compared to 2 cases (18.2%) in Grade I (p<0.001). On the other hand, no significant correlation was detected between CD44 expression and other variables as age, sex, recurrence rate and brain invasion in studied cases (p>0.05).
The correlations of CD44 expression with the clinicopathologic variables in the studied meningioma cases
| Variables | Total no. | CD44 expression | p-value |
|---|
| Mild | Moderate | Marked |
|---|
| Age | ≤50 | 19 | 11(52.40%) | 5(62.50%) | 3(27.30%) | 0.256 |
| >50 | 21 | 10(47.60%) | 3(37.50%) | 8(72.70%) | |
| Sex | Female | 27 | 14(66.7%) | 5(62.5%) | 8(72.7%) | 0.889 |
| Male | 13 | 7(33.3%) | 3(37.5%) | 3(27.3%) | |
| Grade | I | 24 | 20(95.2%) | 2(25.0%) | 2(18.2%) | <0.001 |
| II, III | 16 | 1(4.8%) | 6(75.0%) | 9(81.8%) | |
| Recurrence | Negative | 35 | 20(95.2%) | 6(75.0%) | 9(81.8%) | 0.190 |
| Positive | 5 | 1(4.8%) | 2(25.0%) | 2(18.2%) | |
| Brain invasion | Negative | 35 | 20(95.2%) | 6(75.0%) | 9(81.8%) | 0.190 |
| Positive | 5 | 1(4.8%) | 2(25.0%) | 2(18.2%) | |
Different histologic variants of meningioma cases were enrolled in this study and correlated with CD44 expression as shown in [Table/Fig-2].
CD44 expression in different histologic variants in the studied meningioma cases;
| Histologic variants | CD44 expression |
|---|
| Mild | Moderate | Marked |
|---|
| Meningiothelial | 7(33.30%) | 0(0.00%) | 0(0.00%) |
| Fibroblastic | 4(19.0%) | 0(0.00%) | 1(9.10%) |
| Transitional | 6(28.60%) | 2(25.0%) | 1(9.10%) |
| Angiomatous | 1(4.80%) | 0(0.00%) | 0(0.00%) |
| Microcystic | 1(4.80%) | 0(0.00%) | 0(0.00%) |
| Psammomatous | 1(4.80%) | 0(0.00%) | 0(0.00%) |
| Clear cell | 1(4.80%) | 0(0.00%) | 0(0.00%) |
| Atypical | 0(0.00%) | 5(62.50%) | 7(63.60%) |
| Anaplastic | 0(0.00%) | 1(12.50%) | 2(18.20%) |
The Ki-67 proliferative activity ranged from 0.4% to 30% with average 6.5%. Correlation between Ki-67 expression and clinicopathologic variables is summarized in [Table/Fig-3]. Significant positive correlation was detected between Ki-67 proliferative activity and meningioma grade (p<0.001) and brain invasion (p=0.033). Other variables as age, sex, and, recurrence rate of meningioma didn’t reveal significant correlation with Ki-67 proliferative activity (p>0.05).
The correlation of Ki-67 proliferative activity with clinicopathologic variables in the studied meningioma cases.
| Variables | Total no. | Ki-67 | p-value |
|---|
| Mean | SD |
|---|
| Age | ≤50 | 19 | 6.8 | 8 | 0.835 |
| >50 | 21 | 6.3 | 7.9 | |
| Sex | Female | 27 | 5.5 | 7.2 | 0.248 |
| Male | 13 | 8.6 | 8.9 | |
| Grade | I | 24 | 2.9 | 2.2 | <0.001 |
| II | 13 | 10.4 | 9.4 | |
| III | 3 | 19.0 | 11.5 | |
| Recurrence | Negative | 35 | 5.8 | 7.4 | 0.109 |
| Positive | 5 | 11.8 | 9.9 | |
| Brain invasion | Negative | 35 | 5.5 | 6.8 | 0.033 |
| Positive | 5 | 13.5 | 11.8 | |
A significant positive correlation was detected between CD44 immunohistochemical expression in meningioma cases and Ki-67 proliferative index as shown in [Table/Fig-4].
The correlation of CD44 expression with Ki-67 proliferative activity in studied meningioma cases.
| Variables | CD44 expression |
|---|
| Mild | Moderate | Marked | |
|---|
| Mean | SD | Mean | SD | Mean | SD | p-value |
|---|
| Ki-67 | 3.2 | 2.4 | 8.8 | 8 | 11.3 | 11.5 | 0.011 |
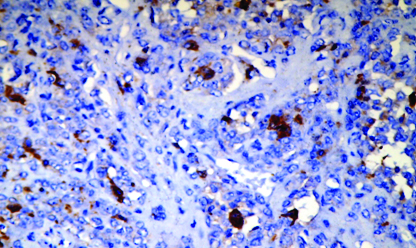
Representative figures for CD44 and Ki 67 expression in meningioma cases are shown in [Table/Fig-5,6 and 7] and [Table/Fig-8,9] respectively.
Mild CD44 immunohistochemical expression in meningioma (20X HPF).
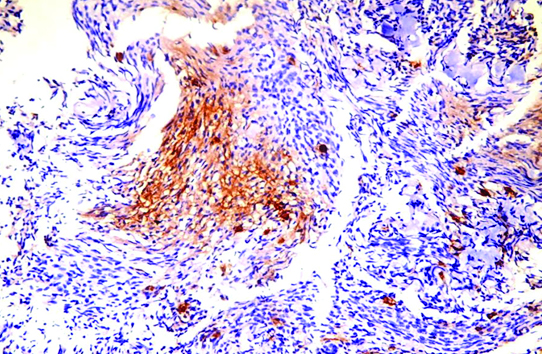
Moderate CD44 immunohistochemical expression in meningioma (10X HPF).
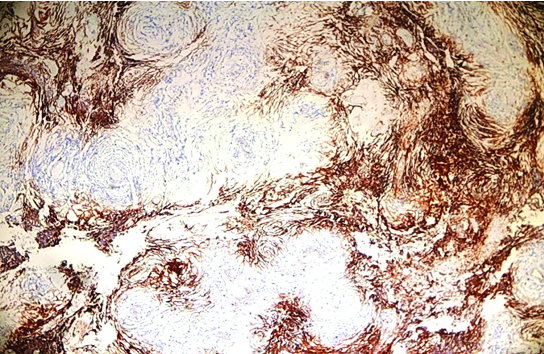
Marked CD44 immunohistochemical expression in meningioma (40X HPF);
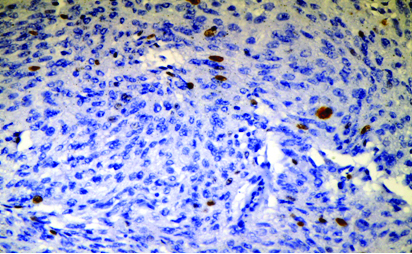
Low Ki-67 proliferative activity in meningioma (20X HPF).
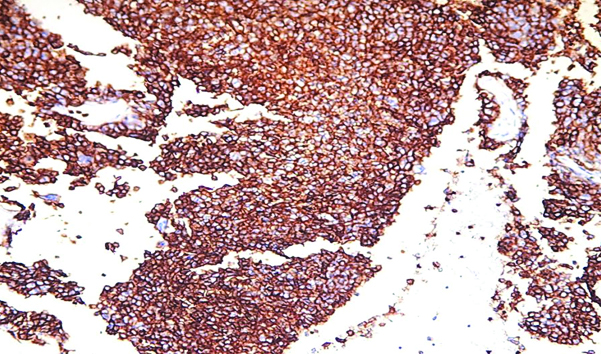
High Ki-67 proliferative activity in meningioma (20X HPF).
Discussion
CD44, a member of cell adhesion molecule family is a cell surface glycoprotein that has a variety of functions, such as promoting cell proliferation, migration and transmitting survival signals [21]. In addition, CD44 is the first identified Cancer Stem Cell (CSC) marker accounting for tumour progression in multiple cancers [22,23]. Subsequently, many current researches investigated the role of this marker in CSCs detection and targeted cancer therapy [24,25].
In the current study, we aimed to investigate the immunohistochemical expression of CD44 and Ki67 in forty cases of meningioma and to highlight their possible correlation with all clinicopathologic variables. Moreover, we aimed to elucidate the role of CD44 as CSC marker in meningioma proliferation through correlating the results of CD44 expression with Ki67 proliferative activity.
The results of this study showed that most meningioma patients were females (27/40) with female:male ratio reported 2.1:1. This figure was similar to that reported by Gassoum et al., where females constituted 61.9% of their patients with female:male ratio 1.6:1 [20].
Twenty one (52.5%) patients in our study were more than 50-year-old, while 19 patients (47.5%) were younger than 50 years. Slightly lower figures were reported by Gassoum et al., where 52.4% of their meningioma patients were in the age group 41 to 50 years [20].
CD44 and Ki67 immunohistochemical expression in meningioma cases were correlated with variable clinical data available in this work as patients’ age and sex but without any statistical significance reported as p>0.05.
In the present work, CD44 and Ki67 were significantly correlated with meningioma grade; CD44 was markedly expressed in 11 meningioma cases; two cases (18.2%) were meningioma grade I and, 9 cases (81.8%) were high grade meningioma II and III which was statistically significant (p<0.001). These results coincide with study done by Trenda et al., and Arsene et al., where a significant parallel increase (p<0.05) in CD44 expression in high grade meningioma compared to benign meningioma were reported [26,27].
The mean Ki-67 expression reported in this study was 2.9%, 10.4% and 19.0% in meningioma grade I, II and III respectively with statistical significance (p<0.001). Arsene D et al., reported almost near values as Ki-67 labelling index was 3.8% in grade I meningioma, 13.42% in grade II meningioma and 18% in grade III meningioma in their study [27].
CD44 was expressed in different histologic variants of meningiomas enrolled in this work as mild CD44 expression was mostly detected in benign grade I meninigiomas mostly in meningiothelial variant (33.3%), and only in one clear cell grade II meningioma (4.8%), while moderate CD44 expression was detected mostly in atypical grade II meningioma (62.5%) but only in 1 anaplastic grade III meningioma (12.5%) and 2 transitional grade I meningioma (25%). Marked CD44 expression was highly reported also in grade II meningioma (63.3%), followed by grade III anaplastic meningioma (18.2%) then finally grade I as 1 fibroblastic (9.1%) and 1 transitional meningioma (9.1%). These figures were similar to those reported by Gassoum et al., on Sudanese patients where strong CD44 expression was mostly reported in atypical grade II meningioma (100%) [20]. Arsene D et al., [27], didn’t report a specific association with any histological variant in the benign category which was also in agreement with our results. Trenda et al., similarly reported strong CD44 expression in atypical meningiomas, but differed in other variants results as no expression in fibroblastic meningiomas and moderate expression in the other subtypes of benign meningiomas were reported in their study [26].
In the current study, Ki-67 was significantly correlated (p=0.033) with brain invasiveness, suggesting that parenchymatous brain invasion is a marker of aggressive behaviour in meningioma. However, no significant correlation was reported regarding CD44 expression and brain invasion (p=0.190). Figarella-Branger et al., reported similar results regarding CD44 expression and brain invasiveness as CD44 was expressed in 4 out of 5 meningiomas demonstrating brain invasion without statistical significant relation mentioned in their study [10].
In our study, no significant correlation was detected between CD44 or Ki67 expression in meningioma cases and rate of tumour recurrence (p=0.476 and, p=0.109 respectively). However, a shift towards higher Ki-67 proliferative activity was noted in recurrent meningiomas (mean Ki-67 11.8%) than non- recurrent ones (mean Ki-67 was 5.8%). This was similar to Abramovich and Prayson study where higher Ki-67 proliferative activity was reported in recurrent meningiomas but without statistical significance (p=0.057; r=0.261) [15]. Also, this was in agreement with Arsene et al., results, where they reported that there was no particular expression of cell adhesion molecules including CD44 in the case of recurrent grade I meningioma included in their study [27].
Ki-67 expression was significantly associated with CD 44 expression in our study (p=0.01) which supports the role of CD44 in tumour proliferation and detection of aggressive behaviour in meningioma. This was in agreement with Arsene et al., where a shift toward parallel increase in the CD44 scores with Ki-67 expression was also clear, even though didn’t reach statistical significance (p=0.057; r=0.261) [27].
Thus, the combined evaluation of CD44 and Ki67 proliferation index can be extremely helpful in some borderline cases that don’t clearly fulfil the latest World Health Organization (WHO) histological criteria in diagnosing high grade meningioma. Moreover, CD44 targeted therapy in high grade meningioma should be investigated thoroughly as in breast cancer clinical trials.
Limitation
The main limitation of this study was lacking the correlation of CD44 and Ki 67 expression in different grades of meningioma with survival rate which we recommend in future studies.
Conclusion
In our study, we concluded that CD44 is a marker of aggressiveness in meningiomas as it was significantly highly expressed in grade II and III meningiomas and was positively correlated with higher Ki-67 proliferation indices. Moreover, Ki-67 proliferation activity was significantly correlated with higher meningioma grades and brain invasiveness. Therefore, researches should be carried out to identify the role of CD44 targeted therapy in atypical and anaplastic meningiomas as done in other tumours e.g., breast cancer.