Pre-analytical Errors at the Chemical Pathology Laboratory of a Teaching Hospital
Marlene A Tapper1, James C Pethick2, Lowell L Dilworth3, Donovan A McGrowder4
1 Senior Resident, Chemical Pathology, Department of Pathology, Faculty of Medical Sciences, The University of the West Indies, Mona, Kingston 7, Jamaica.
2 Biomedical Scientist, Department of Chemical Pathology and Metabolic Medicine, University Hospitals of Leicester NHS Trust, Leicester Royal Infirmary, Leicester, United Kingdom.
3 Lecturer and Consultant Chemical Pathologist, Department of Pathology, Faculty of Medical Sciences, The University of the West Indies, Mona, Kingston 7, Jamaica.
4 Senior Lecturer and Head, Sub-Department of Chemical Pathology, Department of Pathology, Faculty of Medical Sciences, The University of the West Indies, Mona, Kingston 7, Jamaica.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Marlene A Tapper, Senior Resident, Department of Chemical Pathology, University of the West Indies, Mona, Kingston 7, St. Andrew, Jamaica.
E-mail: teeanmarl@gmail.com
Introduction
The Chemical Pathology Laboratory at the University Hospital of the West Indies (UHWI) processes specimens received from inpatients, the outpatient department and other medical facilities in Jamaica. Specific rejection criteria are used to determine samples unsuitable for analysis. It has been noted that despite efforts to reduce the number of unacceptable samples received in the laboratory, the problem persists.
Aim
The study seeks to provide empirical evidence of the inadequacies from which improvements can be formulated.
Materials and Methods
Errors recorded in the rejection log in the Chemical Pathology laboratory at the University Hospital of the West Indies for the period were assessed. The types and frequency of errors were determined manually. The yearly rejection ratios over a four-year period were evaluated.
Results
The most common causes for rejection were unlabelled samples (37%), incorrectly labelled specimens (23%), samples submitted in an inappropriate tube (14%) and incomplete or inaccurately completed requisition forms (14%). The rejection ratio for 2015-2016 was 2.1%.
Conclusion
The laboratory must initiate programmes directed at improving the preanalytical process in order to ensure patient safety.
Pre-examination errors, Sample processing, Standard practices
Introduction
The clinical laboratory has a significant role in the provision of timely and accurate results essential to patient management in clinical practice. In order to achieve these goals it is necessary that standard practices are executed at all levels for guarantee optimal benefit to the patient. Standard procedural guidelines for the pre-examination, the examination (analytical) and post-examination stages of processing must be outlined and complied with in order to prevent errors. A commitment to adherence to the procedures can considerably minimise the consequences of inaccuracies which vary from inappropriate treatment to death of patients.
Of the three phases of sample processing the pre-analytical phase has been confirmed to be accountable for up to two-thirds of errors [1,2]. With technological advancement the analytical phase has been considerably improved to presently being liable for less than 15% of errors [3,4] while between 20% and 50% are attributed to inaccuracies at the post-analytical level [4,5].
Pre-examination errors occur prior to, during and post acquisition of the sample before analysis. The first step in assuring quality results begins with performing the right test on the correct patient at appropriate timing [6]. Before sample collection patients may be inadvertently misidentified, the test(s) ordered are unsuitable, the patient is inadequately prepared or the requisition form is unsatisfactorily completed. During sample acquisition inefficiencies include the use of an incorrect tube, insufficient sample collection, improper sample timing, poor collection technique, incorrect order of draw and sample acquisition proximal to an infusion site. After collection the sample can be unlabelled, improperly transported (long in-transit time and ineffective storage temperature), haemolysed, improperly processed prior to analysis and, in the case of anticoagulated specimens, clotted [7]. These processes in the pre-examination phase are largely outside the control of the laboratory, however, considering the importance of reliable results the laboratory must be involved at all levels to maintain credibility. Processes outside the laboratory can be considered as the pre-pre-analytical phase and those occurring in the laboratory, the pre-analytical phase [8]. During the laboratory phase, sources of error include the breakage of samples during centrifugation and accidental spillage prior to analysis.
The medical intern is primarily responsible for the acquisition of blood samples from hospitalised patients at this hospital. Nurses or Patient Care Assistants (PCAs) collect urine samples as ordered by medical doctors. Specimens acquired by invasive procedures are mostly performed by resident medical doctors or by interns under supervision. The clinical clerkship for medical students includes a ten week period in Pathology and Microbiology where comprehensive instructions in laboratory processes and an emphasis on its importance in achieving optimum patient outcome are provided. Regardless, the occurrence of errors persists and continues to increase, ultimately resulting in higher healthcare costs, unnecessary trauma to or delayed treatment of the patient and possibly death.
The laboratory receives specimens primarily from the wards of the hospital, a 579 bed hospital. Prior to receipt in the laboratory these samples may either be placed in a central area on each ward where they are retrieved at various times during the course of the day by porters and brought to the Central Accessioning Area (CAA) of the laboratory, or may be delivered immediately by the interns when urgent results are required. Sample collection from outpatients is performed in the phlebotomy section then transported at intervals by hand to the CAA. Reference services are also offered by the laboratory to other medical facilities for tests that they do not perform in-house. These specimens are taken directly to the CAA. In the CAA, the information on specimens are verified with that on requisition forms. Patient information and test requests are then uploaded to the LIMS (Laboratory Information Management System) after which barcodes are assigned. Patient data on request forms are also matched to records in the database to prevent duplication and to identify errors. The CAA personnel centrifuge and aliquot clotted samples and distribute all specimens to the relevant sections; Chemical Pathology, Haematology and Microbiology. Non-conformances are logged and forwarded to the respective departments.
The Chemical Pathology Laboratory processes blood, plasma, serum, urine, stool, calculus, Cerebrospinal Fluid (CSF), pleural fluid and other body fluids. Plain and anticoagulated tubes are used for blood sample collection. Serum from clotted samples collected in plain tubes represent the primary specimen used. Whole blood anticoagulated with potassium Ethylenediaminetetraacetic Acid (EDTA) is used for haemoglobin A1c (HbA1c) while plasma from the EDTA tube is used for the analysis of Adrenocorticotrophic Hormone (ACTH). Glucose is analysed from plasma obtained by centrifugation of samples anticoagulated with sodium fluoride/EDTA. Urine samples may be spot, unpreserved voids or 24-hour collections which may or may not contain preservatives depending on the analyte required. Other specimens are collected in sterile universal containers. The present study aims to identify pre-analytical errors which results in rejection of samples in the clinical laboratory and seek solutions to limit inaccuracies.
Materials and Methods
A retrospective analysis was done assessing the pre-analytical errors recorded in the Chemical Pathology Laboratory from June 2012 to May 2016. The Chemical Pathology Laboratory records all errors in a “sample rejection” log noting the date, time, order number, the origin of the sample, type of sample, name of the patient, reason for rejection and the action taken. Reasons for rejection were classified into four groups: Inappropriate specimen (IS); inappropriate requisition form (IF); inappropriate sample volume (IV) and inappropriate collection tube (IT). The data was manually assessed.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Results
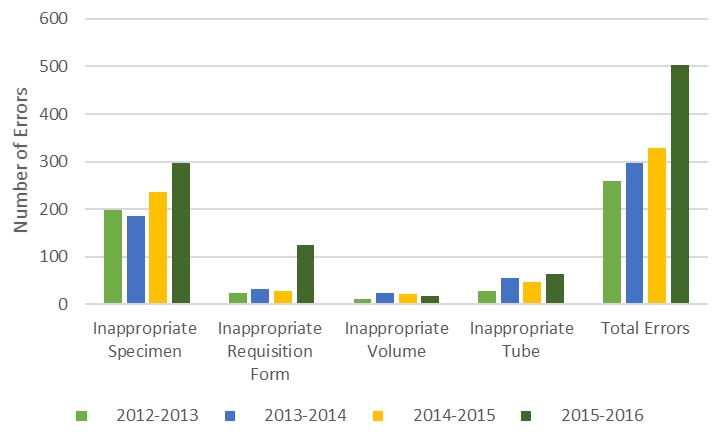
All requests with errors were rejected. Pre-examination errors recorded yearly, from June to May indicated that from 2012 to 2013 the number of rejections was 259, a total of 296 was recorded from 2013 to 2014, 331 from 2014 to 2015 and 504 from 2015 to 2016. Rejections were further categorised as depicted in [Table/Fig-1].
Categories of pre-examination errors.
| 2012-2013 | 2013-2014 | 2014-2015 | 2015-2016 |
|---|
| Inappropriate Specimen |
| Incorrect labelling | 30 | 35 | 78 | 183 |
| Unlabelled | 134 | 126 | 147 | 106 |
| Specimen grossly haemolysed | 28 | 13 | 3 | 2 |
| Specimen unsuitable for analysis | 7 | 11 | 5 | 6 |
| Specimen old | 0 | 0 | 3 | 1 |
| Inappropriate Requisition Form |
| Incomplete/inaccurate information | 19 | 28 | 23 | 124 |
| No tests requested | 2 | 1 | 2 | 0 |
| Form bloodstained | 2 | 3 | 2 | 0 |
| Inappropriate Volume |
| Sample leaked in transit | 2 | 4 | 2 | 5 |
| Sample broken in transit | 3 | 7 | 4 | 0 |
| No specimen received | 0 | 1 | 2 | 5 |
| Insufficient quantity (QNS) | 2 | 5 | 5 | 6 |
| Sample broken/spilled in laboratory | 3 | 6 | 8 | 2 |
| Inappropriate Tube |
| Incorrect sample for test | 27 | 56 | 47 | 64 |
| Total Errors | 259 | 296 | 331 | 504 |
For the entire study, 513 (37%) samples were mostly unlabelled, 326 (23%) incorrectly labelled, 194 (14%) submitted in an inappropriate tube for the requested test and 194 (14%) accompanied by an incomplete or inaccurately completed requisition form [Table/Fig-1]. The distribution of errors by groups is illustrated in [Table/Fig-2].
Comparison of the distribution of pre-examination errors recorded per year.
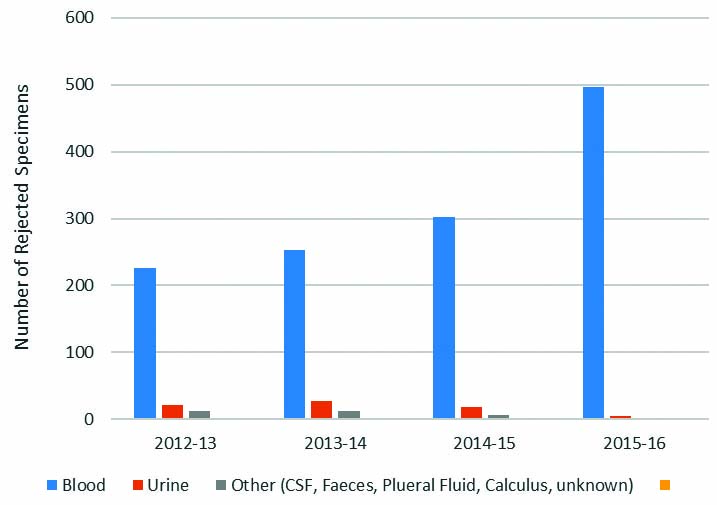
The majority of rejections overall, 1,279 (92%), was associated with blood samples as expected since this was the sample used for the most common analytes processed in clinical chemistry. Other samples included urine, 72 (5%), and CSF, faeces, pleural fluid and calculi, 34 (2%). The sample types and number of rejections are given in [Table/Fig-3].
Number of rejections by types of specimen.
Information for the total number and types of specimens received were unavailable for the periods covering June 2012 to May 2015. However, there were 23,922 samples received from June 2015 to May 2016. The total errors recorded were 504 and represented a 2.1% rejection rate.
Discussion
For the period of the study, unlabelled specimens, incorrectly labelled specimens, incorrect tubes and incomplete or inaccurate request forms comprised the majority of errors recorded. Total errors have trended upward yearly. Unlabelled samples is a common and serious omission at the hospital accounting for the most significant reason for sample rejection with 134 (52%) in 2012-2013, 126 (43%) in 2013-2014 and 147 (44%) in 2014-2015. In 2015-2016, the number of errors due to unlabelled specimens {106 (21%)}, was exceeded by those involving incorrect labelling of samples {183 (36%)}. Number of errors due to incomplete or inaccurate information on the request form was 124 (25%). Errors with labelling (incorrectly labelled and unlabelled) for 2015 to 2016 resulted in a rejection rate of 1.2%. The total rejection rate for the period was 2.1%. Chawla R et al., recorded 1.52% total errors [9] in a study done at the clinical chemistry laboratory of the Govind Ballabh Pant Hospital, a tertiary care, 600 bed hospital in New Delhi, India, while Begum F demonstrated a rate of 5.2% [10] at a 2185 bed hospital in Assam, India.
Morrison A et al., reported labelling errors at a rate of 0.04% to 0.1%. Rates were significantly reduced, up to 43% in the population studied, with the introduction of automated systems [11]. The most common cause of rejection noted in another study was haemolysis followed by inadequate sample volume [9] and is contrary to our findings and that of a study previously done by Dilworth LL et al., at this institution [12].
Laboratory results are essential in the management of patients. Errors can negatively impact outcome and also result in increased cost of healthcare. We are committed to implementing programmes for continued education in order to reduce and possibly eliminate errors. The laboratory has to maintain its integrity by ensuring that accurate and reliable results are reported and therefore, must take the initiative to safeguard all steps of sample processing. The implementation of automated systems involving the generation of request forms with patient identification and matching the uploaded information on barcoded wristbands, can significantly decrease the number of errors observed in labelling [11]. Labels could be printed at the point of sampling and immediately attached to samples after phlebotomy. The University Hospital is now in the process of implementing a Hospital Information Management System (HIMS), however, until then efforts have to be directed to perfecting the system presently utilised.
With automated systems, information regarding the types of samples, the tube and specific conditions required for individual tests could also be easily accessed via an intranet. In the meantime, the laboratory must make an effort to educate and re-educate the relevant personnel involved in the form of continuing education by lectures and making available documents that outline standard procedures. Medical students on the Clinical Pathology rotation must be targeted since they later become interns responsible for the collection of specimens from inpatients and the majority of specimens received in the laboratory are from the wards. The observation of a progressive increase in the number of errors each year also warrants corrective action. Policies to be introduced include tutorials dedicated to the examination of pre-analytical factors affecting laboratory tests and a compulsory in-lab observation of the specimen reception process to improve awareness of the causes of sample rejection. Continuing medical education credits will be offered constantly on the subject.
The laboratory must implement policies to maintain the integrity of the pre-analytical phase for patient safety and also for the requirement of the accreditation process. The International Organization for Standardisation (ISO) 15189:2012 [13] provides guidelines for assuring result validity which may be used for preparation of standard procedures. The laboratory is currently preparing for accreditation and it must ensure that all procedures are standardised for accuracy of results.
Limitation
The total number of specimens received in the laboratory from June 2012 to May 2015 was unavailable; therefore rejection ratios could not be calculated. The information pertaining to samples collected from inpatient/outpatients was not reported due to incomplete records.
Conclusion
The trend shows an increase in pre-analytical errors over the study period. The onus is on the laboratory to reduce this by the provision of standardised guidelines for the pre-analytical phase of testing regardless of the fact that supervision of this process is not a direct responsibility of the laboratory. Ongoing education to improve awareness will also be implemented.
[1]. Hilborne L, Choosing wisely: selecting the right test for the right patient at the right timeMLO Med Lab Obs 2014 46(5):40 [Google Scholar]
[2]. Atay A, Demir L, Cuhadar S, Saglam G, Unal H, Aksun S, Clinical biochemistry laboratory rejection rates due to various types of preanalytical errorsBiochemia Medica 2014 24(3):376-82. [Google Scholar]
[3]. Magee L, Preanalytical variables in the chemistry laboratoryLab Notes 2005 15(1):1-4. [Google Scholar]
[4]. Plebani M, Laboratory errors: How to improve pre- and post-analytical phasesBiochemia Medica 2007 17(1):5-9. [Google Scholar]
[5]. Carraro P, Plebani M, Errors in a stat laboratory: types and frequencies 10 years laterClin Chem 2007 53:1338-42. [Google Scholar]
[6]. Plebani M, The detection and prevention of errors in laboratory medicineAnn Clin Biochem 2010 47:101-10. [Google Scholar]
[7]. Kaushik N, Green S, Pre-analytical errors: their impact and how to minimize themMLO Med Lab Obs 2014 46(5):22,24,26 [Google Scholar]
[8]. Plebani M, Errors in clinical laboratories or errors in laboratory medicine?Clin Chem Lab Med 2006 44:750-59. [Google Scholar]
[9]. Chawla R, Goswami B, Identification of the types of preanalytical errors in the clinical chemistry laboratory:1 year study at GB Pant HospitalLab Medicine 2010 41:89-92. [Google Scholar]
[10]. Begum F, A study of preanalytical errors in a hospital based clinical biochemistry laboratory and formulation of measures for correctionInt J Bioassays 2014 3(9):3270-75. [Google Scholar]
[11]. Morrison A, Tanasijevic M, Goonan E, Lobo M, Bates M, Lipsitz S, Reduction in specimen labelling errors after implementation of a positive patient identification system in phlebotomyAm J Clin Pathol 2010 133:870-77. [Google Scholar]
[12]. Dilworth LL, McGrowder DA, Thompson RK, Identification of pre-examination errors in the chemical pathology laboratory at the University Hospital of the West IndiesIndian J Clin Biochem 2014 29(2):227-31. [Google Scholar]
[13]. ISO 15189:2012. Medical laboratories – requirements for quality and competence. https://www.iso.org/standard/56115.html [Google Scholar]