Amelanotic Signet Ring Cell Melanoma Presenting as Breast Lump- A Diagnostic Conundrum
Prasath Sathiah1, Debasis Gochhait2, Subathra Adithan3, Sandhya Umamahesweran4, Priyadarshini Dehuri5
1 Senior Resident, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
2 Assistant Professor, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
3 Assistant Professor, Department of Radiodiagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
4 Junior Resident, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
5 Senior Resident, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Debasis Gochhait, Assistant Professor, Department of Pathology, Institute Block, JIPMER-605006, Puducherry, India.
E-mail: debasis.go@gmail.com
Amelanotic signet ring cell melanoma is one of the rare variants of malignant melanoma. Here we are presenting a case of a 58-year-old female with chief complaints of swelling in the left sternal region/breast, and right cervical region. Contrast Enhanced CT scan showed the two well circumscribed lobular mass lesions with central necrosis in the left breast. The radiologist opined the lesions as intramammary nodes. Biopsy from the larger breast mass lesion showed a tumour with cells arranged in discohesive pattern less with hetrogenos morphology. These tumour cells had a predominantly signet ring morphology along with markedly pleomorphic tumour cells and giant cells. These tumour cells were negative for pan CK and positive for S100, HMB45. So the case was diagnosed as metastatic amelanotic malignant melanoma with signet ring morphology.
Lymph node, Immunohistochemistry, Metastasis, S100
Case Report
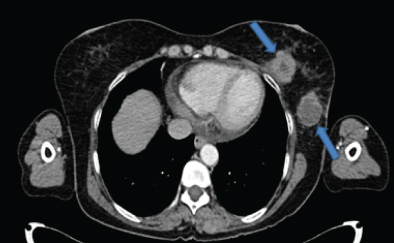
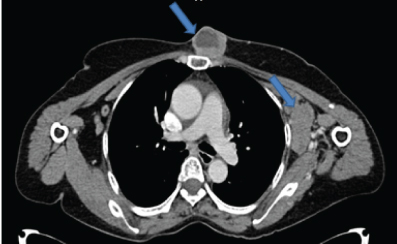
A 58-year-old female presented to the surgery OPD with chief complaints of lump in the left breast region and right cervical lymphadenopathy. The clinician suspected a possibility of carcinoma breast with distant metastasis. On radiological evaluation (CECT neck and thorax) there were two circumscribed lobulated heterogeneously enhancing intramammary lesions (largest lesion measured 4.5 x 3.6 cm in size with central non-enhancing necrotic area) in the lower quadrant closely abutting the pectoral muscles in the left breast [Table/Fig-1]. In addition another heterogeneously enhancing mass of 4.7 x 3.3 x 3 cm size with central non-enhancing necrotic soft tissue attenuating lesion was noted in the pre-sternal region at the level of manubrio-sternal joint [Table/Fig-2]. There was no focal lesion in the right breast. Multiple discrete and conglomerate nodes in the bilateral axillary and right cervical level II, III and V and left cervical level II and V were also seen. The patient also had mild pericardial effusion however there was no bony involvement. The final radiology opinion was suggestive of a lymphomatous involvement of the nodes/metastatic nodes. Peripheral blood smear examination showed features of iron deficiency anaemia, no atypical lymphoid cells were identified.
Contrast CT axial section through lower chest shows left intramammary necrotic lesions (arrows);
Contrast CT axial section just below manubrium sterni shows presternal and left axillary nodes lesion (arrows).
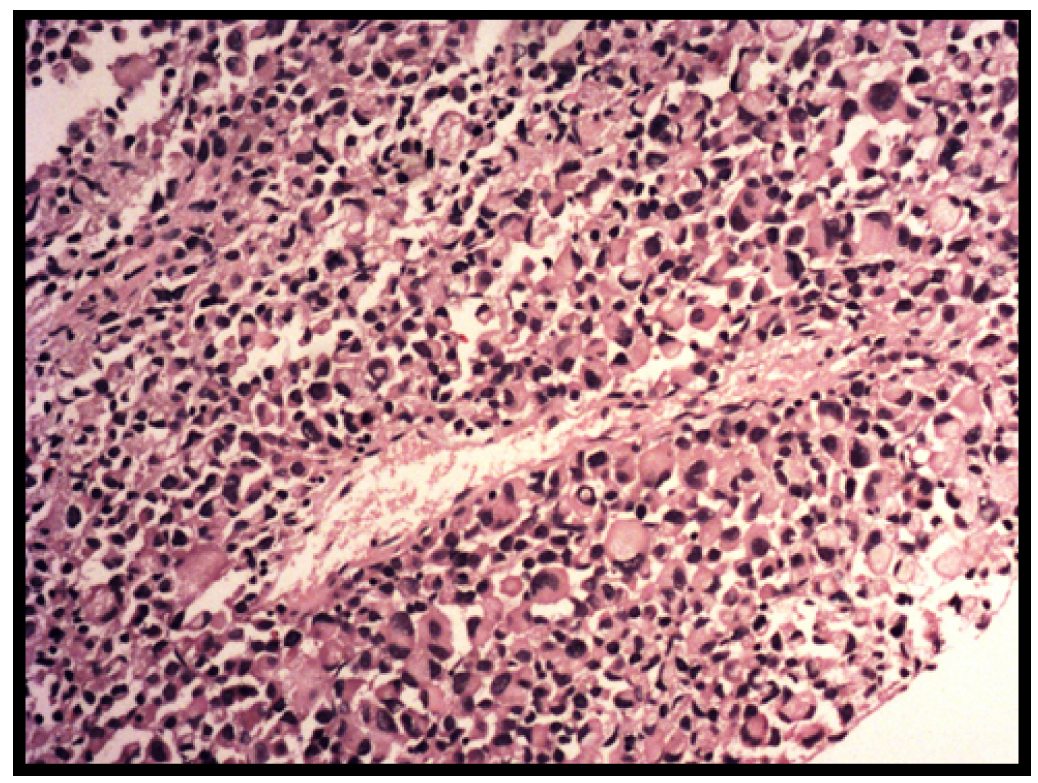
Subsequently the patient underwent trucut biopsy from the larger lesion on left breast. H and E stained section showed a dyscohesive pattern of tumour cells with heterogeneous morphology. The tumour cells showed a predominance of signet ring cell morphology along with many binucleated and multinucleated forms. Prominent eosinophilic nucleoli were seen in the non signet ring cells while few of the tumour cells showed intra-nuclear inclusions [Table/Fig-3,4]. Area with tumour necrosis and scattered lymphocytes were seen in the periphery of the tumour and around the blood vessels. Because there was a history of breast lump a possibility of a pleomorphic infiltrating ductal carcinoma/lobular carcinoma or a metastatic carcinoma was considered as the initial histopathological diagnosis with a request for Pan CK, E cadherin and GCDFP immunohistochemistry. In addition to that a mucicarmine stain performed for mucin was also negative. As these markers were negative a second panel of vimentin, CK 7, LCA, CD 138, CD 68 and S 100 were requested. To our surpise only vimentin and S 100 was positive, so a third panel of HMB 45 and Melan A was performed to confirm the tumour as melanoma [Table/Fig-5]. Scattered lymphoid tissue was seen in theperiphery of core biopsy and taking into consideration the multiple lesions in the intramammary and presternal region we suggested a diagnosis of nodal metastatic amelanotic melanoma with signet ring cell morphology. Mean while patient had undergone FNAC from the cervical lymph nodes which showed tumour cells with similar morphology. On detailed clinical examination no primary site for the melanoma could be detected. She was planned for a PET CT scan followed by treatment with dacarbazine; however she was lost to follow up.
Core biopsy from the left breast shows tumour cells arranged in sheets exhibiting predominantly signet ring cell morphology with abundant eosinophilic cytoplasm (H and E, 100x).
Biopsy from left breast tumour shows: a) intranuclear inclusion (H and E, 40X); b) intracytoplasmic inclusion like structure which compressing and displacing the nucleus to the periphery (H and E, 100X).
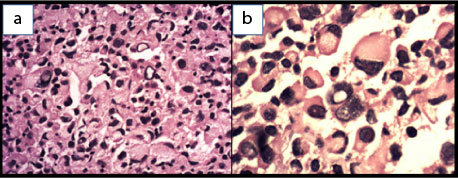
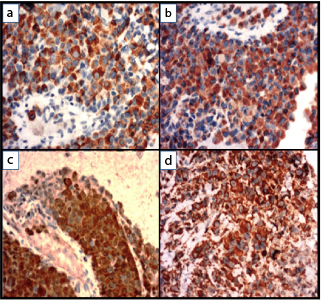
Immunohistochemistry shows tumour cells are positive for: a) HMB45; b) Melan A; c) S100, d) Vimentin (IHC, 40X).
Discussion
Malignant melanomas can exhibits a wide range of microscopic morphological appearances. Some of the rare types of malignant melanomas are clear cell, chondroid, osteoid, myxoid, rhabdoid and small cell type [1]. They can cause confusion with many other malignant tumours which have this morphology. Two morphological subtypes of clear cells can be seen in melanoma, one is the signet ring cell type (single large vacuole) and the other type is balloon cell type (abundant cytoplasm with multiple vacuoles) [2].
Signet ring melanoma is defined as a melanoma with more than 50% tumour cells having signet-ring shaped nuclei [3]. Review of the clinico pathological features of 23 previously reported cases of signet ring melanoma showed that middle aged males are commonly affected by this subtype. Most of the cases presented as skin lesions (n=13), followed by lymphnodes (n=5), gastrointestinal tract (n=2) and single cases each was reported in the ovary, peritoneal effusion, lung. Among all these cases 10 cases were reported as metastasis, 2 were recurrences, 9 were reported as primary and 2 cases were not specified [4]. The Index case is a 58-year-old lady with the clinical presentation as breast lump and an unknown primary site.
Signet ring morphology is usually focal but may be diffuse throughout the tumour and this subtype was mostly seen in recurrent or metastatic lesions of malignant melanoma [5]. Our case also showed prominent signet ring morphology in the metastatic site (intramammary node). Because of the unusual morphology and lack of melanin pigment, this tumour was confused with many epithelial tumours. Among the cutaneous neoplasms, the presence of signet ring cells is usually reported in metastatic adenocarcinoma, melanocytic nevi, malignant melanoma, squamous cell carcinoma, basal cell carcinoma, hidradenoma, malignant lymphoma, liposarcoma, cylindroma, infundibular cyst, plasmacytoma and epithelioid smooth muscle tumours [6].
Intracytoplasmic inclusions of signet ring melanoma appears as eosinophilic granular or fibrillar with an ill-defined border in the surrounding cytoplasm. In contrast in metastatic adenocarcinomas these inclusions are slightly bluish have more vacuolated and distinct border with the surrounding cytoplasm. In addition nuclei of the signet ring melanoma showed binucleation, more pleomorphism with vesicular chromatin and prominent eosinophilic nucleoli which is not usual in signet ring carcinoma. Dyscohesion and lack of glandular pattern is a common feature of both signet ring carcinoma and signet ring cell melanoma [5]. In difficult cases, simple mucin stain such as PAS, PAS-D, alcian blue or mucicarmine can be of real help. Immunohistochemistry cytokeratin should be performed to confirm the epithelial nature of the tumours in all cases with signet ring cell morphology. Vimentin stain showed positivity in 100% of signet ring cell melanoma. PAS or PAS-D stain was not very useful because some cases (22% cases) of signet ring melanoma may show PAS-positive cytoplasmic inclusions. So if the tumour was negative for mucin stain and pan-cytokeratin further immunohistochemistry with melanocytic markers were mandatory to exclude the possibility of signet ring melanoma [5]. According to the review of previously reported cases, these tumour cells were positive for S100 (90% cases), Melan-A (100% cases) and HMB45 (89.5% cases). However, there are exceptions to this pattern exists as some of these tumours were negative for S100 and HMB45. Staining with cytokeratin is usually negative in all the cases except for one case which showed weak positive staining [5].
Focally some of the cells were showing relatively round nuclei with less peripherally located nuclei. These cells raise the possibility of the rhabdoid type of melanoma. These rhabdoid cells have polygonal shape with round nuclei, open chromatin, prominent nucleoli and abundant cytoplasm. Immunohistochemistry showed decreased expression of S100 or loss of expression of HMB45 or both were seen [7]. Our case showed strong expression of S100 and HMB45. The presence of multiple empty vacuoles within the cytoplasm with scalloping of nucleus imparts a pseudolipoblastic appearance and also metastasis malignant melanoma simulating soft tissue sarcoma may have pleomorphic cells or myxoid stroma [8]. Shanks JH and Banerjee SS found that CD 38 immunohistochemistry marker was expressed in alarge proportion of malignant melanoma, so it was not useful in differentiating melanoma from plasmacytoma [9]. Our case showed negativity for CD138. Few of the lymphoma also can exhibit signet ring morphology. The majority of which were B-cell lymphoma, follicular lymphoma and few were T- cell lymphoma and anaplastic large-cell lymphoma [10-12]. Our case was negative for Leucocyte Common Antigen (LCA).
Some authors considered signet ring appearance was a poor prognostic factor because most of the cases were commonly presented with metastasis. But some other authors have found that signet ring cells were also described in melanocytic nevi, so it may not be the marker of biological behaviour [13,14]. Present case presented with extensive metastasis in bilateral cervical and intramammary nodes and the primary was still not ascertained even after extensive investigation.
Conclusion
Malignant melanoma is the greatest mimicker in pathology which always poses a problem in cytology and histopathology. Amelanotic signet ring melanoma is the rare unusual variant of melanoma which can easily lead to a misdiagnosis if the interpreting expert is not aware or suspicious of these unusual variants. At least an S-100 immunochemistry can be helpful in suspecting a melanoma when the tumour shows signet ring cells along with tumour giant cells and multinucleated cells even in the absence of pigment.
[1]. Sheibani K, Battifora H, Signet-ring cell melanoma a rare morphologic variant of malignant melanomaAm J Surg Pathol 1988 12:28-34. [Google Scholar]
[2]. Rütten A, Huschka U, Requena C, Rodríguez-Peralto JL, Requena L, Primary cutaneous signet-ring cell melanoma: a clinico-pathologic and immuno-histochemical study of two casesAm J Dermatopathol 2003 25(5):418-22. [Google Scholar]
[3]. Nakhleh RE, Wick MR, Rocamora A, Swanson PE, Dehner LP, Morphologic diversity in malignant melanomasAm J Clin Pathol 1990 93:731-40. [Google Scholar]
[4]. Kocovski L, Alowami S, Signet ring cell melanoma: a potential diagnostic pitfallAm J Dermatopathol 2014 36(12):985-88. [Google Scholar]
[5]. Grilliot MA, Goldblum JR, Liu X, Signetring cell melanoma of the gastroesophageal junction: a case report and literature reviewArch Pathol Lab Med 2012 136(3):324-28. [Google Scholar]
[6]. Bastian BC, Kutzner H, Yen TSB, LeBoit PE, Signet-ring cell formation in cutaneous neoplasmsJ Am Acad Dermatol 1999 41:606-13. [Google Scholar]
[7]. Abbott JJ, Amirkhan RH, Hoang MP, Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literatureArch Pathol Lab Med 2004 128(6):686-88. [Google Scholar]
[8]. Lodding P, Kindblom LG, Angervall L, Metastases of malignant melanoma simulating soft tissue sarcoma. A clinico-pathological, light- and electron microscopic and immunohistochemical study of 21 casesVirchows Arch A Pathol Anat Histopathol 1990 417(5):377-88. [Google Scholar]
[9]. Shanks JH, Banerjee SS, VS38 immunostaining in melanocytic lesionsJ Clin Pathol 1996 49(3):205-07. [Google Scholar]
[10]. Harris M, Eyden B, Read G, Signet ring cell lymphoma: a rare variant of follicular lymphomaJ Clin Pathol 1981 34(8):884-91. [Google Scholar]
[11]. Weiss LM, Wood GS, Dorfman RF, T-cell signet-ring cell lymphoma. A histologic, ultrastructural, and immunohistochemical study of two casesAm J Surg Pathol 1985 9(4):273-80. [Google Scholar]
[12]. Falini B, Liso A, Pasqualucci L, Flenghi L, Ascani S, Pileri S, CD30+anaplastic large-cell lymphoma, null type, with signet-ring appearanceHistopathology 1997 30(1):90-92. [Google Scholar]
[13]. Won JH, Ahn SK, Lee SH, Lee WS, Kim SC, Signet-ring cell melanoma: poor prognostic factor?Br J Dermatol 1994 131(1):135-37. [Google Scholar]
[14]. LiVolsi VA, Brooks JJ, Soslow R, Johnson BL, Elder DE, Signet cell melanocytic lesionsMod Pathol 1992 5(5):515-20. [Google Scholar]