Introduction
Hypothyroidism is a common concern in endocrinology practice, which plays a significant role in metabolic and development processes. Obesity, hyperlipidaemia and hypertension may complicate hypothyroidism. Recent studies have shown that cytokines like leptin and adiponectin, secreted by adipose tissue and exert their endocrinal functions by modulating appetite, obesity and insulin sensitivity in conjunction with thyroid hormones. Interrelation between thyroid hormone, insulin resistance and adipokines are not yet clear.
Aim
To estimate serum leptin, adiponectin and insulin resistance in patients with hypothyroidism and to compare with control subjects and measure the relation between the mean value of one variable with others.
Materials and Methods
Forty primary hypothyroidism patients and forty age and sex matched controls were selected for the study with informed consent. Fasting serum Thyroid Stimulating Hormone (TSH), leptin, adiponectin, glucose and insulin were estimated. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was evaluated from fasting plasma glucose and serum insulin levels. Statistical analysis was carried out using SPSS version 17.0. Unpaired t-test and regression analysis were used to compare and determine the dependence, p<0.05 was considered significant.
Results
Serum TSH, leptin, adiponectin HOMA-IR were significantly higher (p<0.05) in patients with hypothyroidism (10.37±4.10, 10.97±0.60, 31.09±4.07, 3.64±0.40) than controls (2.41±2.09, 10.37±0.12, 33.32±1.44, 2.36±0.35). Regression analysis showed that leptin was significantly (p=0.054) dependent on adiponectin but not on others.
Conclusion
Increased oxidative stress by hypothyroid mediated leptin secretion and increased insulin resistance can down-regulate the adiponectin secretion and future complications. Serum estimation and correction of imbalance of adipokines in hypothyroidism can prevent severe consequences.
Adiponectin, HOMA-IR, Leptin, Thyroid stimulating hormone
Introduction
Thyroid hormones and Thyroid Stimulating Hormone (TSH) are the fine regulators of intermediate metabolism in our body. They affect the response of other hormones and the metabolism of carbohydrate, protein and lipids [1,2]. So, any alteration in thyroid profile will affect the metabolic spectra which ultimately disturb appetite, body weight, muscle mass and adipose tissue and can also contribute in the pathogenesis of insulin resistance, type 2 diabetes and cardiovascular diseases [3].
Recent studies have shown that cytokines like leptin and adiponectine, which are exclusively secreted by adipose tissue, perform an endocrinal function in conjunction with thyroid hormones. Leptin, the `ob` gene product and a lipostatic hormone by nature [4,5], is critical for the regulation of Thyroid Thyrotropin Releasing Hormone (TRH) gene expression in the paraventricular nucleus of the hypothalamus for normal functioning of the thyroid axis [6]. Serum leptin concentration is increased in obesity, insulin resistance and dyslipidaemia and as it increases the oxidative stress and calcification in vascular endothelium, it can be a confounding factor for atherosclerosis [7].
Adiponectin is expressed by transcript 1 (apM1) gene and exhibits anti-atherogenic and anti-inflammatory properties [8,9] Its serum level is decreased in obesity, type-2 diabetes, dyslipidaemia and cardiovascular diseases [10,11].
Therefore, thyroid dysfunction can affect the functions of adipose tissue and together they can alter the metabolism and energy homeostasis. But the inter-relationship between thyroid function and adipose tissue secreting cytokines is not yet clear [12].
Hypothyroidism and hyperthyroidism are the two ends of thyroid disorders. Forty-two million people in India suffer from thyroid disorder [9]. Prevalence of hypothyroidism is 3.9% and it is higher in women than men [13].
In hypothyroidism, increased TSH and decrease in T3 and T4, results in increased of body weight and alteration of lipid profile. These changes are associated with glucose and insulin metabolism along with adipose tissue metabolism [14,15]. Alteration of thyroid profile may affect leptin and adiponectin secretions and can lead to further complications.
The aim of our present study was to compare and correlate serum fasting leptin and adiponectin levels and their effect on insulin resistance in hypothyroid patients when compared to healthy subjects.
Materials and Methods
This cross-sectional, observational and non-interventional study was carried out in the Department of Biochemistry, Calcutta National Medical College, Kolkata, India. The duration of study was six months (March 2016 to August 2016). The study was approved by the Institutional Ethical Committee as per the Helsinki Declaration and Informed consent was taken from the study participants.
Inclusion criteria: A total of 40 hypothyroid (16 males and 24 females, aged 30 to 60 years) patients were selected for the study from the Thyroid Clinic of the College. Forty age and sex matched control subjects were also selected, with consent.
Exclusion criteria: Excluded from this study were those with active neoplasm or history of neoplasm, other endocrine disorders like diabetes mellitus, severe liver dysfunction, renal failure, hypertension, diseases of the pituitary gland or hypothalamus including secondary hypothyroidism, major surgery within two weeks of enrolment, a severe psychiatric condition, GIT malabsorption diseases, pregnancy, alcohol abuse, concurrent medication that may interfere with thyroid hormone alterations, critically ill patients, Hashimoto’s thyroid disease and thyroidectomized patients on L-T4 treatment.
Methods for analysis of test parameters: A total of 5 ml of blood from the subjects was collected aseptically using standard protocols. The serum was separated by centrifugation (3000 rpm for five min) immediately and analysis was done.
Estimation of serum TSH was done by sandwich Enzyme-linked Immunosorbent Assay (ELISA) (Aspen Laboratories Pvt. Ltd.,) [16].
Estimation of serum leptin and adiponectin were done by sandwich ELISA (RayBiot, Inc) [17,18].
Plasma glucose was analyzed by using reagent kit (Merck) by glucose oxidase method [19]. Serum insulin was measured by ELISA with monoclonal antibody based reagent (Monobind) [20]. HOMA-IR was calculated by the formula: Fasting insulin × Fasting glucose/405.
Statistical Analysis
Analysis was done by the Statistical Package for the Social Sciences (SPSS) version 17.0. Results were expressed in mean±SD, dependence of various parameters in case group. Unpaired t-test and regression analysis were done. The p<0.05 was considered as statistically significant.
Results
[Table/Fig-1] shows distribution of mean, SD and mean SEM of various parameters in case and control groups. Mean value of TSH, leptin, HOMA-IR is increased in case group than control whereas the adiponectin is decreased in case group.
Shows distribution of mean, Standard Deviation (SD) and Standard Errors of Mean (SEM) of various parameters in case and control groups.
| Parameters | n | Mean± SD | SEM |
|---|
| TSH (micro IU/ml) Case | 40 | 10.37± 4.10 | 0.6483 |
| TSH (micro IU/ml) Control | 40 | 2.41± 2.09 | 0.3305 |
| Leptin (pg/ml) Case | 40 | 10.37± 0.12 | 0.0190 |
| Leptin (pg/ml) Control | 40 | 10.97±0.60 | 0.0949 |
| Adiponectin (pg/ml) Case | 40 | 31.09± 4.07 | 0.6435 |
| Adiponectin (pg/ml) Control | 40 | 33.32±1.44 | 0.2277 |
| HOMA –IR Case | 40 | 3.64±0.40 | 0.0632 |
| HOMA –IR Control | 40 | 2.36±0.35 | 0.0553 |
[Table/Fig-2] shows comparison of mean differences in various parameters between patients with hypothyroidism and controls. There was a significant difference of mean TSH, leptin, adiponectin and HOMA-IR between the cases and controls (p<0.001). Mean of TSH, leptin, HOMA-IR were significantly increased in case group than control whereas the adiponectin was significantly decreased in case group.
Shows comparison of mean differences in various parameters between hypothyroids and controls.
| Parameters | t | df | p |
|---|
| TSH | 10.9396 | 78 | 0.001 |
| Leptin | 6.0984 | 78 | 0.001 |
| Adiponectin | 3.2669 | 78 | 0.001 |
| HOMA–IR | 15.2311 | 78 | 0.001 |
Dependent Variable: Leptin (case)
* indicates p significant
[Table/Fig-3] shows linear regression analysis of leptin as a dependent variable. It was observed that leptin is significantly dependent on adiponectin in hypothyroid patients (p=0.054) whereas, the dependence is not statistically significant in case of HOMA-IR or TSH in the same.
Shows linear regression analysis of leptin as a dependent variable.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. |
|---|
| B | Std. Error | Beta |
|---|
| (Constant) | 16.043 | 8.411 | | 1.907 | 0.068 |
| HOMA-IR (Case) | 0.822 | 2.210 | 0.067 | 0.372 | 0.713 |
| Adipo (Case) | -0.079 | 0.039 | -0.383 | -2.014 | 0.054* |
| TSH (Case) | -0.021 | 0.237 | -0.017 | -0.090 | 0.929 |
[Table/Fig-4] shows the dependence between leptin and adiponectin in patients with hypothyroidism. It was marked that leptin is significantly dependent on adiponectin.
Showing the dependance between leptin and adiponectin in hypothyroids.
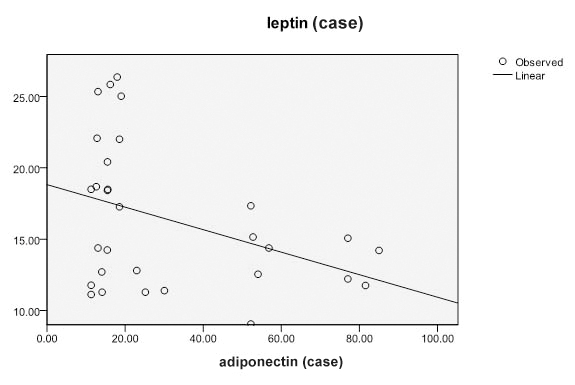
Discussion
In the present study, we found a significant increase in serum leptin and HOMA-IR in patients with hypothyroidism than in controls, whereas the serum adiponectin level showed an opposite trend.
Our findings are similar with the findings of Chen Y et al., which corresponds with a previous study [21] which showed that insulin resistance was associated with hypothyroidism [3]. Some other authors, however, have not found any change in leptin and HOMA-IR in hypothyroid patients [21,22].
We found increased leptin concentrations in hypothyroid patients when compared to controls. Variations in plasma TSH contributes to the regulation of leptin pulses by regulating its mRNA expression [6,21] along with stimulating adipocytes directly to secrete leptin in peripheral tissue [22].
Insulin resistance develops when the efficacy of insulin on various organs like liver, muscle and adipose tissue decreases. A deficiency or an excess of thyroid hormones has been demonstrated to induce development of insulin resistance and disruption of glucose metabolism [3,23]. In hypothyroidism, peripheral insulin resistance develops, whereas in hyperthyroidism, hepatic and peripheral insulin resistance is observed [3,24,25]. The aetiology may be “altered expression of glucose transporter on monocytes” [25] and changes of same in the peripheral blood circulation [26]. The observed increase in HOMA-IR in hypothyroid patients is thus explained.
Adiponectin may increase the thyroid hormone synthesis, specially f-T3 as the C-terminal globular structure of adiponectin interacts with the gC1q receptor of thyroid cell mitochondria [27].
On regression analysis, we found that leptin is significantly dependent (negative) on adiponectin in hypothyroid patients whereas the dependence is not statistically significant in HOMA-IR or TSH in the same patients. Hotta K et al., found an inverse relationship between leptin and adiponectin in non- insulin dependent diabetes mellitus in rhesus monkey [10]. Individuals with obesity, insulin resistance and diabetes are usually present with decreased circulating levels of adiponectin [29,30].
We found decreased trend of adiponectin concentrations whereas opposite trend was found in leptin concentration and insulin resistance in hypothyroid patients.
Leptin promotes angiogenesis and atherogenesis by generating oxidative stress in endothelial cells whereas adiponectin acts as anti-atherogenic and anti-inflammatory agent by inhibiting TNF-α. It is also an insulin sensitizer [9].
While treating a hypothyroid patient, not only the thyroid status should be taken care of but the confounding factors should also be considered to prevent the associated complications. As hypothyroidism itself alters the lipid profile, it makes the patient more prone to atherosclerosis. Moreover, decrease in adiponectin concentrations can increase the atherogenic mechanism by increasing leptin. Furthermore, decreased insulin sensitization can ultimately lead to cardiovascular risk and metabolic syndrome. In metabolic syndrome both the regions on chromosome 3q27 where the gene encoding adiponectin is located and the regions on chromosome 17p12 that are strongly linked to plasma leptin concentrations are affected [28]. From our study, we can hypothesize that increased oxidative stress produced by increased leptin concentrations and HOMA-IR, may cause gradual inactivation of adiponectin.
Limitation
Small sample size is the major limitation of the study. We recommend studies to be conducted with large study subjects to know the effect of thyroid on various metabolic diseases.
Conclusion
Conditions like obesity, dyslipidaemia, coronary atherosclerosis, Coronary Heart Disease (CHD) may complicate hypothyroidism. Cytokines like leptin, adipokines are strongly correlated with the levels of thyroid hormone and insulin. We analysed serum leptin, adiponectin and calculated insulin resistance. Serum leptin, HOMA-IR level were significantly high and adiponectin was significantly low in primary hypothyroid patients than in controls. Regression analysis showed the leptin was significantly dependent on adiponectin in the case group. We can conclude that the measurement of these parameters in hypothyroidism can predict and prevent the future serious consequences of the disease process.
Dependent Variable: Leptin (case)* indicates p significant
[1]. Krotkiewski M, Thyroid hormones in the pathogenesis and treatment of obesityEur J Pharmacol 2002 440:85-98. [Google Scholar]
[2]. Potenza M, Via MA, Yanagisawa RT, Excess thyroid hormone and carbohydrate metabolismEndocr Pract 2009 15:254-62. [Google Scholar]
[3]. Gierach M, Gierach J, Junik R, Insulin resistance and thyroid disordersEndokrynol Pol 2014 65:70-76. [Google Scholar]
[4]. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, Positional cloning of the mouse obese gene and its human homologueNature 1994 372:425-32. [Google Scholar]
[5]. Mantzoros CS, The role of leptin in human obesity and disease: a review of current evidenceAnnals of Internal Medicine 1999 130:671-80. [Google Scholar]
[6]. Flier JS, Harris M, Hollenberg AN, Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiringJournal of Clinical Investigation 2000 105:859-61. [Google Scholar]
[7]. Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL, Leptin enhances the calcification of vascular cells: artery wall as a target of leptinCirculation Research 2001 88:954-60. [Google Scholar]
[8]. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K, cDNA cloning and expression of a novel adipose specific collagen-like factor, apMI (adipose most abundant gene transcript 1)Biochemical and Biophysical Research Communications 1996 221:286-89. [Google Scholar]
[9]. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappa B signaling through a c-AMP-dependent pathwayCirculation 2000 102:1296-301. [Google Scholar]
[10]. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Plasma concentrations of a novel adipose specific protein, adiponectin, in type 2 diabetic patientsArteriosclerosis, Thrombosis and Vascular Biology 2000 20:1595-99. [Google Scholar]
[11]. Matsubara M, Maruoka S, Katayose S, Decreased plasma adiponectin concentrations in women with dyslipidemiaJour of Clinical Endocrinol and Metabol 2002 87:2764-69. [Google Scholar]
[12]. Zimmermann T, Brabant G, Holst JJ, Feldt-Rasmussen U, Circulating leptin and thyroid dysfunctionEuropean Jour of Endocrinol 2003 149:257-71. [Google Scholar]
[13]. Unnikrishnan AG, Menon UV, Thyroid disorders in India: An epidemiological perspectiveIndian J Endocrinol Metab 2011 15(Suppl 2):78S-81S. [Google Scholar]
[14]. Menon VU, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian populationJour Indian Med Assoc 2009 107:72-77. [Google Scholar]
[15]. Pucci E, Chiovato L, Pinchera A, Thyroid and lipid metabolismInt J Obesity Rel Metab Dis 2000 24:109S-13S. [Google Scholar]
[16]. Schall RF, Fraser AS, Hansen HW, Kern CW, Teneso HJ, A Sensitive manual enzyme immunoassay for thyroxineClinical Chemistry 1978 24(10):1801 [Google Scholar]
[17]. Youseef M, The effect of dietary intervention by low carbohydrate diet, and low fat diet on weight loss, leptin and adiponectinLife Sc Jour 2015 12(4):33-42. [Google Scholar]
[18]. Tsao TS, ACRP30, A new hormone controlling fat and glucose metabolismEuropean Journal of Pharmacol 2002 440(2-3):213-21. [Google Scholar]
[19]. Raabo E, Terkildsen TC, The enzymatic determination of blood glucoseScand Clin Lab Invest 1960 12:402-07. [Google Scholar]
[20]. Andersen L, Dinesen B, Jørgensen PN, Poulsen F, Røder ME, Enzyme immunoassay for intact human insulin in serum or plasmaClinical Chemistry 1993 39:578-82. [Google Scholar]
[21]. Chen Y, Wu X, Wu R, Sun X, Yang B, Wang Y, Changes in profile of lipids and adipokines in patients with newly diagnosed hypothyroidism and hyperthyroidismScientific Reports 2016 6:26174 [Google Scholar]
[22]. Corbetta S, Englaro P, Giambona S, Persani L, Blum WF, Beck-Peccoz P, Lack of effects of circulating thyroid hormone levels on serum leptin concentrationsEur J Endocrinol 1997 137:659-63. [Google Scholar]
[23]. Seven R, Thyroid status and leptin in Basedow-Graves and multinodular goiter patientsJ Toxicol Environ Health 2001 63:575-81. [Google Scholar]
[24]. Guo F, Bakal K, Minokoshi Y, Hollenberg AN, Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivoEndocrinology 2004 145:2221-27. [Google Scholar]
[25]. Menendez C, Baldelli R, Camina JP, Escudero B, Peino R, Dieguez C, T.S.H. stimulates leptin secretion by a direct effect on adipocytesJour of Endocrinol 2003 176:7-12. [Google Scholar]
[26]. Havekes B, Sauerwein HP, Adipocyte-myocyte crosstalk in skeletal muscle insulin resistance;is there a role for thyroid hormone?Curr Opin Clin Nutr Metab Care 2010 13:641-46. [Google Scholar]
[27]. Peppa M, Koliaki C, Nikolopoulos P, Raptis SA, Skeletal muscle insulin resistance in endocrine diseaseJ Biomed Biotechnol 2010 2010:527850 [Google Scholar]
[28]. Mitrou P, Raptis SA, Dimitriadis G, Insulin action in hyperthyroidism: a focus on muscle and adipose tissueEndocr Rev 2010 31:663-79. [Google Scholar]
[29]. Fernandez-Real JM, Lopez-Bermejo A, Casamitjana R, Ricart W, Novel interactions of adiponectin with the endocrine system and inflammatory parametersJour of Clinical Endocrinol and Metab 2003 88:2714-18. [Google Scholar]
[30]. Diekman MJ, Anghelescu N, Endert E, Bakker O, Wiersinga WM, Changes in plasma Low-Density Lipoprotein (LDL) and High Density Lipoprotein (HDL) cholesterol in hypo- and hyperthyroid patients are related to changes in free thyroxine, not to polymorphisms in LDL receptor or cholesterol ester transfer protein genesJ Clin Endocrinol Metab 2000 85:1857-62. [Google Scholar]