Amyloidosis is a term used for diseases caused by the extracellular deposition of insoluble polymeric protein fibrils in the tissue and organs leading to loss of function. Various varieties of amyloid proteins share certain characteristic tinctorial properties like congophilia and green birefringence under polarized light [1].
The deposition of amyloid in previously apparently normal skin without deposits in the internal organs is known as PLCA. Various subtypes of PLCA are recognized, including the more common macular and papular (lichen amyloidosis) types and the rare nodular (tumefactive) form. Both macular and papular lesions can occur in the same patient giving rise to the term biphasic amyloidosis [2].
Clinically, it is difficult to distinguish different subtypes of primary cutaneous amyloidosis. Histopathology of cutaneous amyloidosis using H&E stain shows eosinophilic hyaline material in papillary dermis, which can be further confirmed by CR stain. One of the limitations of CR stain is that it may not detect amyloid in all the cases of cutaneous amyloidosis, especially macular amyloidosis in which amyloid deposition is scant [3].
DIF test for tissue-bound autoantibodies provide a useful adjunct for the diagnosis of primary cutaneous amyloidosis, thereby differentiating clinically and histologically similar dermatological conditions [4]. Amyloid deposits fluoresced positively for immunoglobulins or complements particularly Immunoglobulin M (IgM) or Complement 3 (C3) [5]. Immunohistochemical findings confirm the presence of keratin epitopes in the amyloid of lichen amyloidosis and macular amyloidosis [6].
The goal of the present study was to assess the concordance between the clinical, histopathological and DIF findings in various subtypes of (PLCA). We intend to examine cases of amyloidosis stained with CR stain and see if such a method offers any advantage with respect to immunofluorescence of gamma globulins.
Materials and Methods
In the current observational study, 50 newly diagnosed cases of primary cutaneous amyloidosis, attending the OPD of a tertiary care center were recruited from December, 2012 to June, 2014.
As the prevalence of cutaneous amyloidosis in the literature is 0.2-0.3%, it will require a very large sample size. Therefore, we included all 22,127 patients attending dermatology OPD during the study period of one and half year to have the maximum sample size possible to increase the accuracy of the estimation process [7,8].
All the patients suspected to be suffering from cutaneous amyloidosis on the basis of clinical symptoms and signs were included in the study, after obtaining an informed written consent. Patients having any systemic disease were excluded from the study. The study was approved by Ethical Committee of SS Institute of Medical Sciences and Research Centre, India.
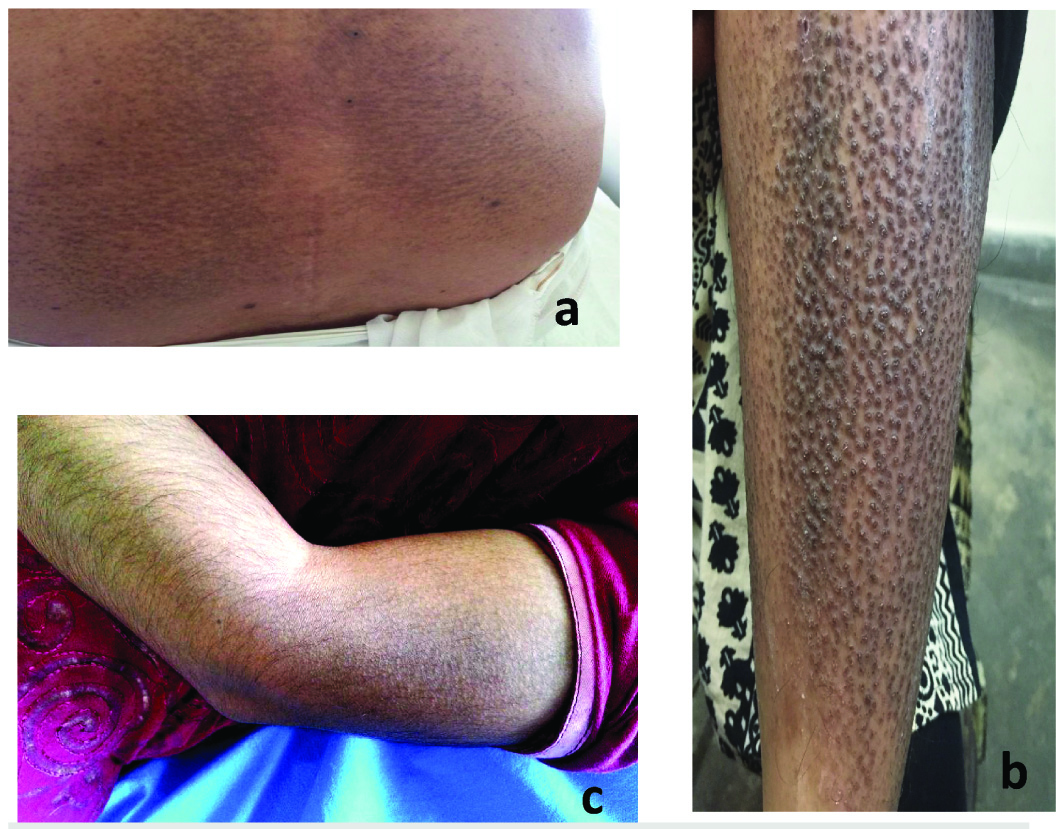
Patients having confluent or reticulate hyperpigmented macular lesions with or without pruritus were labelled as macular amyloidosis. Patients with hyperkeratotic, pea-sized, hyperpigmented, pruritic papules were classified as ‘lichen or papular amyloidosis’. Patients having both the lesions were grouped under ‘Biphasic amyloidosis’ [9,10] [Table/Fig-1a-c].
Clinical presentation of cutaneous amyloidosis: a) Macular amyloidosis showing rippled pigmentation on lower back; b) Lichen amyloidosis showed lichenified papules and nodules with pigmentation on lower limbs; and c) Biphasic amyloidosis showed rippled pigmentation with papules on extensor aspect of arm.
Two punch biopsy skin tissues were taken using disposable punches measuring between 3 mm to 4 mm from 50 selected patients. One set of specimen was fixed in 10% formalin and stained with H&E and CR stain which were examined under light microscopy. Other set of specimen was fixed in Michels stain and was sent for DIF.
Patients were evaluated for age, sex, site, aetiological factors, comorbidities and symptoms. Histopathological and immunofluorescence findings were recorded. Descriptive data was summarized as percentages or means.
Statistical Analysis
Statistical analysis was performed using SPSS computer software (Version 20.0, SPSS Inc, Chicago, IL, USA). Descriptive data was summarized as percentages or means. Sensitivity and specificity of the test was calculated and was used to predict its diagnostic accuracy.
Results
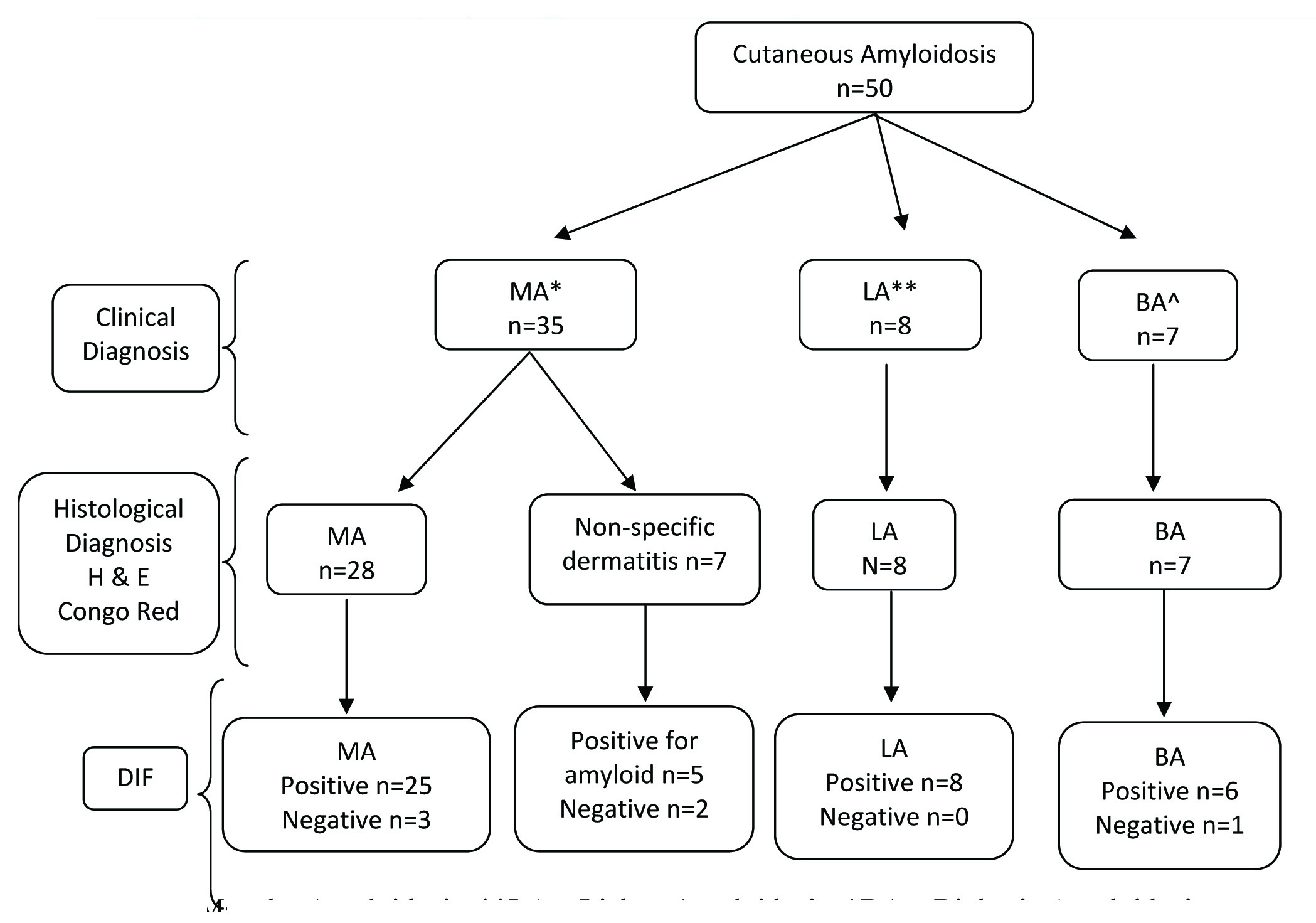
The flow chart showing diagnostic approach for patients with clinical diagnosis of cutaneous amyloidosis enrolled in the present study is shown in [Table/Fig-2]. Patients were evaluated for age, sex, site, aetiological factors, comorbidities and symptoms [Table/Fig-3]. Histopathological and immunofluorescence findings were recorded [Table/Fig-4,5].
Flow chart showing diagnostic approach for cutaneous amyloidosis. *MA – Macular Amyloidosis, **LA – Lichen Amyloidosis, ^BA – Biphasic Amyloidosis
Clinical characteristics of the patient cohort.
| Clinical characteristics | Cases (n = 50) |
|---|
| Age (Mean±SD) in years | 36±11.76 (range, 16-59 years) |
| Sex Ratio (Male:Female) | 1 : 2.57 |
| OccupationHouse wifeStudentOthers | 21(42%)11(22%)18(36%) |
| Positive Family History | 6 (12%) |
| ComorbiditiesDiabetesAcanthosis NigricansMelasmaPsoriasis with keratosis pilaris | 4(8%)1(2%)1(2%)1(2%) |
| AetiologyScrubNo Scrub | 28(56%)22(44%) |
| SymptomsPruritisAsymptomatic | 17(34%)33 (66%) |
| Site of Involvement*Extensor aspect of armUpper backExtensor of forearmNeckPretibialLower BackOthers | 38(76%)29(58%)23 (46%)7 (14%)5 (10%)5 (10%)2 (04%) |
All the patients had multiple sites of involvement
Histopathological characteristics of cutaneous amyloidosis^.
| Histopathological characteristics | Clinical diagnosis | Total (n=50) |
|---|
| MA* (n=35) | LA** (n=8) | BA† (n=7) |
|---|
| N (%) | N (%) | N (%) | N (%) |
|---|
| Acanthosis | 8 (22.8%) | 6 (75%) | 4 (57.1%) | 18 (36%) |
| Hypergranulosis | 1 (2.8%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Hyperkeratosis | 11 (31.4%) | 7 (87.5%) | 3 (42.9%) | 21 (42%) |
| Parakeratosis | 1 (2.8%) | 0 (0%) | 0 (0.0%) | 1 (2%) |
| Elongation of Rete Ridges | 0 (0%) | 4 (50%) | 1 (14.3%) | 5 (10%) |
| Degeneration of Basal cells | 0 (0%) | 7 (87.5%) | 3 (42.8%) | 10 (20%) |
| Atrophy of epidermis | 1 (2.8%) | 0 (0%) | 0 (0.0%) | 1 (2%) |
| Amyloid in papillary dermis | 28 (80%) | 8 (100%) | 7 (100.0%) | 43 (86%) |
| Pigmentary Incontinence | 3 (8.6%) | 3 (37.5%) | 2 (28.6%) | 8 (16%) |
| Inflammatory infiltrate | 8 (22.8%) | 4 (50%) | 3 (42.8%) | 15 (30%) |
MA-Macular Amyloidosis,
LA-Lichen Amyloidosis,
BA – Biphasic Amyloidosis
There are more than one histological feature in multiple cases
Immunofluorescence characteristics of cutaneous amyloidosis.
| Characteristics | Clinical diagnosis | Total (n=50) |
|---|
| MA* (n=35) | LA** (n=8) | BA† (n=7) |
|---|
| N (%) | N (%) | N (%) | N (%) |
|---|
| Deposit | Negative | 5 (14.29%) | 0 (0%) | 1 (14.29 %) | 6 (12%) |
| Amorphous | 24 (68.57%) | 8 (100%) | 6 (85.71%) | 38 (76%) |
| Colloid | 2 (5.71%) | 0 (0%) | (0%) | 2 (4%) |
| Oval | 4 (11.43%) | 0 (0%) | 0 (0%) | 4 (8%) |
| Histopathological Site | Negative | 5 (14.29%) | 0 (0%) | 1 (14.29%) | 6 (12%) |
| Papillary Dermis | 30 (85.71%) | 8 (100%) | 6 (85.71%) | 44 (88%) |
| Negative | 5 (14.29%) | 0 (0%) | 1 (14.29%) | 6 (12%) |
| IgM>A>C3 | 15 (42.86%) | 2 (25%) | 0 (0%) | 17 (34%) |
| Type of Immunoreactants | IgM>A>G>C3 | 2 (5.71%) | 2 (25%) | 0 (0%) | 4 (8%) |
| IgM>C3 | 9 (25.71%) | 2 (25%) | 6 (85.71%) | 17 (34%) |
| IgM>G>C3 | 2 (5.71%) | 2 (25%) | 0 (0%) | 4 (8%) |
| IgM>G>C3, Fibrinogen | 2 (5.71%) | 0 (0%) | 0 (0%) | 2 (4%) |
MA-Macular Amyloidosis,
LA-Lichen Amyloidosis,
BA – Biphasic Amyloidosis
Cutaneous amyloidosis accounted for 0.22% (50/22,127) of the total dermatology outpatients during the study period from December 2012 to June 2014. The mean age of subjects was 36±11.76 years (Mean±SD) (range 16-59 years). Thirty-six out of fifty patients were females (male/female ratio 1/2.6). Majority (42%, 21/50) of patients were housewives by occupation. More than half the patients (56%, 28/50) gave history of using scrub while bathing. Family history was present in six cases. Associated comorbidities included four patients with diabetes mellitus, one patient each of acanthosis nigricans, melasma and psoriasis with keratosis pillaris respectively [Table/Fig-3].
The duration of primary cutaneous amyloidosis ranged from as early as one month to as late as 15 years and the mean duration of the lesions before diagnosis was 40.13±41.92 months (Mean±SD). Majority of the patients presented with discoloration of the skin. Pruritus was the presenting symptom only in 17 cases (34%) [Table/Fig-3].
Most common site of cutaneous amyloidosis was the extensor aspect of arm (76%) followed by upper back (58%). All the patients had multiple sites of involvement. There was no evidence of systemic involvement of amyloidosis clinically or with available laboratory investigations [Table/Fig-3].
Rippled pigmentation and confluent pigmentation were the most common lesions seen in 30 (60%) and 21 (42%) patients respectively. Papules were seen in 16 (32%) patients of cutaneous amyloidosis.
Out of 50 skin biopsies with a clinical diagnosis of primary amyloidosis, 43 cases displayed features of cutaneous amyloidosis on histopathology and were confirmed by CR stain [Table/Fig-6a-c]. There was predominance of macular amyloidosis type (35 out of 50 cases).
Histopathological presentation of cutaneous amyloidosis: a) Macular amyloidosis showing unremarkable epidermis and dermis with small angulated eosinophilic amyloid deposits and melanophages (H&E, x40); b) Lichen amyloidosis showing hyperkeratosis, acanthosis, papillomatosis and elongation of rete ridges in epidermis and dermis showing confluent deposits of amyloid (H&E, x400); and c) Congo red positive amyloid deposits in lichen amyloidosis (Congo Red stain, x200).
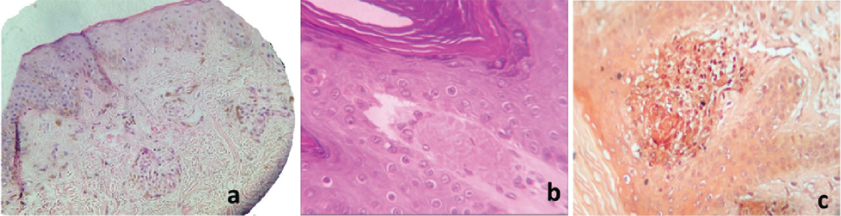
Histopathological examination [Table/Fig-4] of H&E stained sections of 35 clinically diagnosed cases of macular amyloidosis showed small, amorphous globule of eosinophilic material in papillary dermis along with pigmentary incontinence and epidermal changes like hyperkeratosis, acanthosis, hypergranulosis, parakeratosis and atrophy of epidermis in 80% (28/35) cases [Table/Fig-6a]. These deposits were amyloid as confirmed by CR stain. In remaining seven cases, there were ill-defined eosinophilic scanty deposits in the upper dermis which raised a suspicion of amyloid on routine histopathological staining, but CR staining was negative. Thus, a histopathological diagnosis of non-specific dermatitis was offered for these cases. DIF test [Table/Fig-5] detected amyloid in 30 out of 35 (85.71%) clinically diagnosed case of macular amyloidosis. Twenty five (89.28%) histologically confirmed macular amyloidosis cases and 71.4% (five out of seven cases) of non-specific dermatitis cases showed positive immunofluorescence for amyloid in papillary dermis with oval, amorphous and colloid pattern [Table/Fig-7a]. Amyloid was undetectable by both DIF and CR staining method in two cases. The most frequent immunoreactants were IgM, A and C3.
a) Direct immunofluorescence showing green stained oval amyloid deposits in papillary dermis in Macular amyloidosis (Fluorescein Rhodamine Stain, x100); and b) Direct immunofluorescence showing green stained oval amyloid deposits in papillary dermis in lichen amyloidosis (Fluorescein Rhodamine Stain, x200).
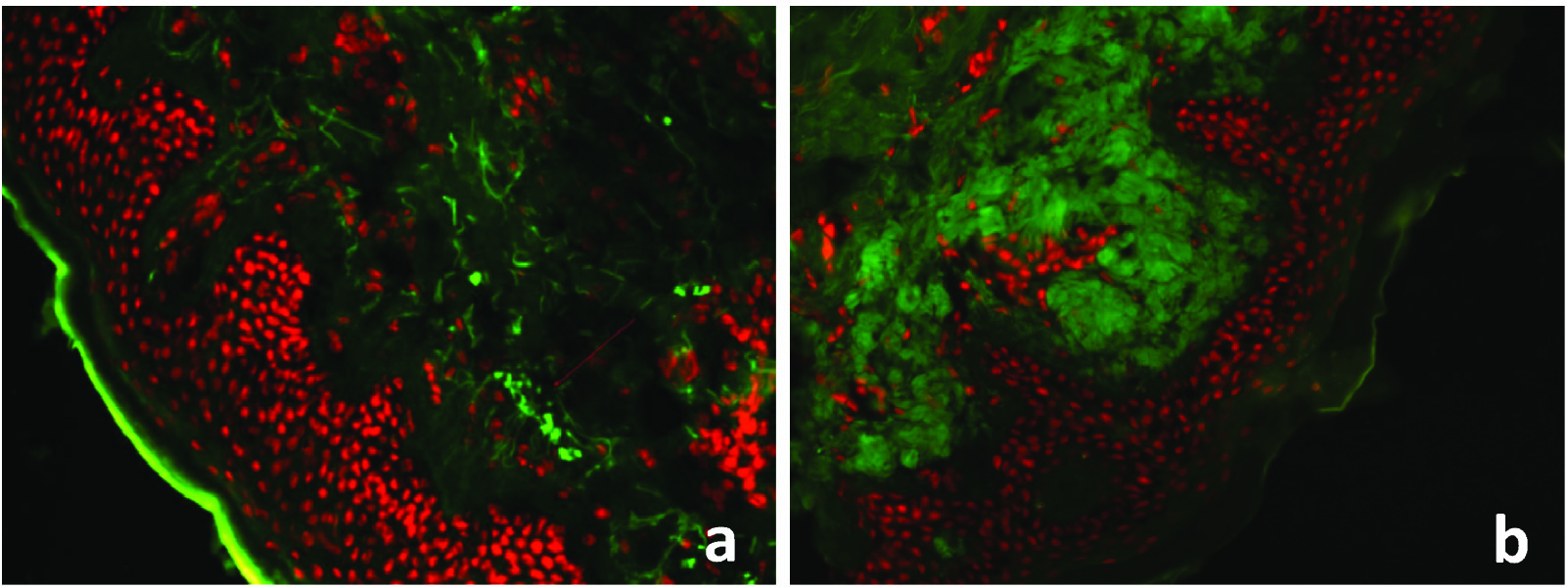
All the clinically diagnosed cases of lichen amyloidosis (8/8,100%) showed large globular deposits of eosinophilic amyloid in papillary dermis [Table/Fig-6b,c]. Following changes were observed – 1) Epidermal: Hyperkeratosis and degeneration of basal cells, acanthosis, elongation of rete ridges; 2) Dermal: Inflammatory infiltrate and pigmentary incontinence. CR staining showed these deposits as reddish orange substances. All cases showed amorphous pattern with IgM, G, A and C3 as immunoreactants on DIF [Table/Fig-7b].
All seven cases of biphasic amyloidosis showed mixed pattern of macular and lichen amyloidosis on histopathology. DIF was positive in 85.71% (6/7) of clinically diagnosed biphasic amyloidosis. No case of nodular amyloidosis was observed.
Of the 35 cases of macular amyloidosis, 28 were CR positive resulting in sensitivity of 84.85% and 30 were DIF positive; resulting in sensitivity of 90.91%. In contrast, seven cases of biphasic amyloidosis showed a sensitivity of 100% and 85.71% with CR and DIF respectively. Thus, the overall sensitivity for cutaneous amyloidosis with CR staining is 89.58% and 91.67% for DIF [Table/Fig-8].
Diagnostic accuracy of Congo Red (CR) and Direct immunofluorescence (DIF).
| Variables | Cutaneous amyloidosis (N=50) |
|---|
| Test | Present | Absent |
|---|
| Congo Red (CR) | |
| Positive | True Positive = 43 | False Positive = 0 |
| Negative | False Negative = 5 | *True Negative =2 |
| Sensitivity = 89.58%, Specificity = 100% |
| Direct Immunofluorescence (DIF) | | |
| Positive | True Positive = 44 | False Positive = 0 |
| Negative | False Negative = 4 | *True Negative = 2 |
| Sensitivity = 91.67%, Specificity = 100% |
True negative cases were those that were negative by both the diagnostic modalities.
Discussion
The diagnostic importance of PLCA is related to its low incidence, its similarity with other diseases and its possible cosmetic concern [11]. A total of 50 patients were clinically diagnosed as cutaneous amyloidosis during one and a half year study period. A similar study conducted in India by Krishna A et al., [12] reported 62 cases during a period of one year. A study done in Saudi Arabia reported 42 clinically suspected cases of PLCA in a period of seven years [7]. This indicated a higher prevalence of PLCA in our setting.
There are geographical variations in various forms of primary cutaneous amyloidosis. In our study, macular amyloidosis was the most common variant at 70%, with lichen and biphasic amyloidosis accounting for 16% and 14% respectively. Macular amyloidosis has a higher incidence in Asia, Middle East and South America [2]. Lichen amyloidosis is a rare skin disorder in Europe and North America, but is common in South East Asia and some South American countries [13]. Genetic factors may also play an important role in the aetiopathogenesis of cutaneous amyloidosis [12].
The present study correlates well with other studies with respect to the occurrence of the disease at a relatively younger age with female predominance [7,14]. We also observed that a history of chronic friction or rubbing of the lesion was an important aetiological factor [15-17]. A familial association of cutaneous amyloidosis was reported in 6/50 (12%) patients in our study, which support the results of other research workers [12,18,19].
Pruritis was the presenting complaint reported by 75% cases of lichen amyloidosis [5,15]. In contrast, patients with macular amyloidosis usually tend to present with asymptomatic discolouration. Apart from distribution of lesions on extensor aspect of arm and upper back, most patients presented with multiple sites of involvement. The distribution reported was similar to that reported by other studies, as these sites were most amendable to friction [3,20]. Contrary to the literature, extensor aspect of arm was the most commonly involved site of lichen amyloidosis in present study [15,21]. Clinically, all the patients presented with hyperpigmentation. In macular amyloidosis, macules coalesced to form rippled or confluent pigmentation; while in lichen amyloidosis, most commonly it was hyperpigmented papules. Lichenification was seen in all the cases of lichen amyloidosis. In cases of biphasic amyloidosis, it was a mixed patterns of macular and lichen amyloidosis [15,21].
In this study, we compared the DIF test and CR staining for the detection of primary cutaneous amyloidosis. Primary cutaneous amyloidosis can be easily diagnosed by a dermatopathologist on H&E stained slides. General pathologists can detect amyloid deposits as extracellular eosinophilic material and CR staining is usually performed for confirmation of diagnosis. Out of 35 clinically diagnosed cases of macular amyloidosis, 28 cases (80%) showed amyloid in papillary dermis which was CR positive; while remaining seven cases that were CR negative were termed as non-specific dermatitis. DIF was able to detect amyloid in five out of seven cases of non-specific dermatitis, thus confirming the diagnosis of macular amyloidosis.
In cases of Lichen amyloidosis, both CR staining and DIF were able to detect amyloid deposits and are thus excellent diagnostic modalities to different lichen amyloidosis from clinically and histologically similar conditions like lichen simplex chronicus and lichen planus pigmentosus. The result of the present study showed that the overall sensitivity of DIF in detecting amyloid was 91.67%, while that of CR was 89.58%.
A study on 30 cases of lichen amyloidosis by Salim T et al., showed fluorescence with IgM, C3 and IgA throughout the basement membrane zone in 100% cases, along with papillary deposits in six (20%) patients, with the intensity of fluorescence being strong for IgM in most of the cases. Our findings of LA were similar to that of Salim T et al., [15].
Habermann MC and Montenegro M in their study on cutaneous amyloidosis found a total of 93.75% gammaglobulins with most frequent being IgG, followed by IgM and C3 in decreasing order in macular amyloidosis cases [22]. Total gammaglobulins were demonstrated in 88.88% of lichen amyloidosis cases with most frequent being IgG, M, A and C3. These findings were quite similar to that observed in our study. Clinico-histopathological discrepancies were observed in three patients only where a clinical possibility other than lichen planus (i.e., dermatomyositis, bullous pemphigoid, and pyostomatitis vegetans) was considered.
MacDonald DM et al., was able to detect immunoglobulins in all cases of PLCA and it helped to differentiate Lichen amyloidosis and lichen planus on the basis of presence of fibrin and fluorescence along basement membrane in lichen planus. Contrary to other studies and our findings [5], Noren P et al., reported negative immunofluorescence with anti-keratin antiserum inspite of large deposits of amyloid in lichen amyloidosis [23]. A study by Fernandez-Flores A concluded that traditional method of staining with CR was inferior to immunohistochemistry for the diagnosis of macular amyloidosis, as amyloid deposit in it were scanty [24].
Limitation
The limitation of our study is small sample size in view of rarity of disease which makes it difficult to make a definitive conclusion. Also, cost and availability of immunofluorescence stain is another major detriment to use of this technique.
Conclusion
DIF is complementary to CR staining for confirming the amyloid deposits in cases of PLCA, especially in macular amyloidosis; While in lichen and biphasic amyloidosis, both the modalities are comparable cases where there is a strong clinical suspicion of amyloidosis, but are negative for CR stain should be confirmed by DIF. CR staining is a simple and cheap technique. If facility is available, DIF can replace CR as a diagnostic technique for amyloidosis, even on a daily basis.
*All the patients had multiple sites of involvement
*MA-Macular Amyloidosis,
**LA-Lichen Amyloidosis,
†BA – Biphasic Amyloidosis
^There are more than one histological feature in multiple cases
*MA-Macular Amyloidosis,
**LA-Lichen Amyloidosis,
†BA – Biphasic Amyloidosis
*True negative cases were those that were negative by both the diagnostic modalities.