GBM is the most common primary malignant tumour of Central Nervous System (CNS) in adults. Treatment outcomes even after multimodal therapies including surgical resection, RT and CT remain very poor [1]. Many studies have been undertaken to improve the management of this aggressive tumour by identifying prognostic factors [2]. This is the first study done in Eastern part of India (Odisha) to assess significance of EGFR in GBM patients. The most frequent alteration of GBM oncogenes consists of over expression of EGFR. EGFR is a transmembrane tyrosine kinase receptor, present in 30%-50% of all GBM cases and more in primary types [3]. Most cases of GBM with EGFR expression exhibit a variety of mutational changes, common one being EGFRvIII [4,5]. Therefore, we assess EGFRvIII expression which plays a dominant role in prognosis of GBM patients.
Normally, activation of EGFR through specific ligands followed by downstream signal cascade activation leads to DNA synthesis and cell proliferation. Therefore, the therapeutic benefit of TMZ depends on its ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues. This methylation damages the DNA and triggers the death of tumour cells [6].
Materials and Methods
This prospective study was carried out in the Department of Pathology and Neurosurgery, SCB Medical College, Cuttack, Odisha, India, between October-2014 and September-2016 and comprises 52 cases of GBM occurring in frontal, parietal, temporal and occipital regions of the brain. The study was undertaken after obtaining Institutional Ethical Clearance and patient consent. All cases of GBM with therapy for which EGFR protein expression was assessed; were included in the study and those without therapy were excluded from the study.
Formaldehyde fixed fifty two biopsy specimens were received and were subjected to routine histopathological examination. Paraffin embedded tissues were sectioned, 3-4 μm by using a microtome (Leica, Germany) and slides were stained with Haematoxylin and Eosin (H&E) stain. All the cases were classified and graded morphologically according to WHO diagnostic criteria for CNS neoplasms [8]. IHC was done by using Anti-EGFR pan kit; Biogenex, Hyderabad, India, following the standard protocol. Squamous cell carcinoma tissue was taken as positive control [Table/Fig-1]. Evident cytoplasmic membrane staining was scored as positive or over expressed. EGFR protein over expression was assessed as a percentage of positive tumour cells in hot spots (10 high power fields). Cases showing >20% expression were considered as EGFR positive [Table/Fig-2a,b] and those having <20% expression as negative.
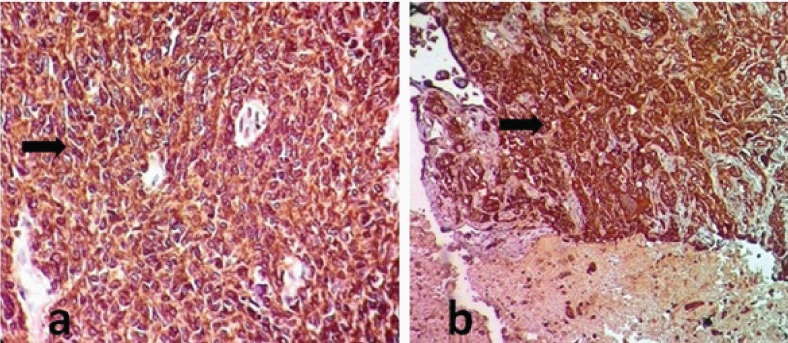
Pictomicrography of positive control: IHC tissue section of squamous cells showing cytoplasmic membrane positivity (EGFR 40X).
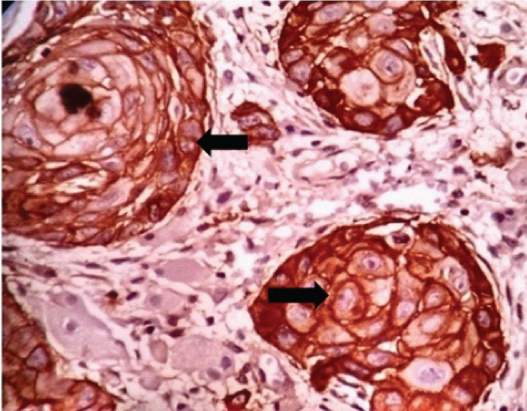
Pictomicrography of IHC showing EGFR positive (a, b): > 20% cytoplasmic membrane positivity (10X and 4X).
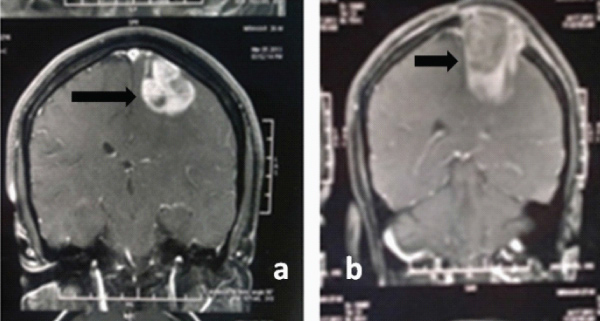
Response to radiotherapy and chemotherapy was evaluated by measuring the tumour volume through Computed Tomography (CT) scan and Magnetic Resonance Imaging (MRI) performed at the time of admission [Table/Fig-3a] and three months after therapy [Table/Fig-3b]. The patients were graded as per RECIST guideline to assess response: Complete Response (CR): 100% reduction, that is disappearance of all target lesions, partial response (PR): >30% reduction in the sum of Longest Diameter (LD) of target lesions; Progressive Disease (PD): >20% increase in the sum of LD of target lesions; Stable Disease (SD): Treatment result lies between PR and PD [7]. All the cases were followed-up at three months intervals till death. All 52 patients covered under this study had died at the time of statistical analysis. Information on patient morbidity and mortality was collected by telephonic/personal means. Morbidity was assessed in terms of Karnofsky Performance Score (KPS) [9]. Overall survival time was defined as the time interval between initial craniotomy and the day of the patient’s death.
MRI scans of 40 years male with GBM in frontal lobe; a) Pre operation; b) Post operation.
Statistical Analysis
Statistical analysis was performed by using IBM-Statistical Package for Social Sciences (SPSS) software, version 20.0 with 5% level of significance.
Results
The mean survival of the patients was calculated using unpaired t-test and ANOVA (analysis of variance) test. The result of this study as highlighted in [Table/Fig-4] showed a mean age of 42.61 years (range, 8-75) with male preponderance. The mean KPS score was 53.5 (range 30-70). Out of 52 cases, 30 cases were EGFR positive (57.7%) and 22 cases (42.3%) were EGFR negative. After surgical resection all cases underwent RT (n-20; 38.5%) or combined RT+CT (n-32; 61.5%). CR, PR, SD and PD were 38.5%, 25.0%, 15.4% and 21.2% respectively. For statistical evaluation CR and PR were categorized as responders (33/52; 63.5%) and group SD and PD as non-responders (19/52; 36.5%). The percentage of responders in the RT+CT group was 3.71% higher and clinically better, compared to radiation group alone.
Association of EGFR protein expression with basic variables and therapy in GBM patients.
| Variables | EGFR Positive (n-30) | EGFR Negative (n-22) | Total | % of patients | p- value |
|---|
| Age (Year) |
| >40 | 16 | 53.30% | 11 | 50.00% | 27 | 51.90% | 0.821 |
| ≦40 | 14 | 46.70% | 11 | 50.00% | 25 | 48.10% |
| Sex |
| Male | 19 | 63.30% | 12 | 54.50% | 31 | 59.60% | 0.523 |
| Female | 11 | 36.70% | 10 | 45.50% | 21 | 40.40% |
| KPS |
| >50 | 15 | 50.00% | 13 | 59.10% | 28 | 53.80% | 0.516 |
| ≦50 | 15 | 50.00% | 9 | 40.90% | 24 | 46.20% |
| tumour site |
| Frontal | 10 | 33.30% | 9 | 40.90% | 19 | 36.50% | 0.313 |
| Parietal | 6 | 20.00% | 8 | 36.40% | 14 | 26.90% |
| Temporal | 10 | 33.30% | 4 | 18.20% | 14 | 26.90% |
| Occipital | 4 | 13.30% | 1 | 4.50% | 5 | 9.60% |
| treatment |
| R+T | 13 | 43.30% | 19 | 86.40% | 32 | 61.50% | 0.002* |
| R | 17 | 56.70% | 3 | 13.60% | 20 | 38.50% |
| Response |
| CR | 6 | 20.00% | 14 | 63.60% | 20 | 38.50% | 0.006* |
| PR | 8 | 26.70% | 5 | 22.70% | 13 | 25.00% |
| SD | 6 | 20.00% | 2 | 9.10% | 8 | 15.40% |
| PD | 10 | 33.30% | 1 | 4.50% | 11 | 21.20% |
| Responders |
| RS | 14 | 46.70% | 19 | 86.40% | 33 | 63.4% | 0.003* |
| NR | 16 | 53.30% | 3 | 13.60% | 19 | 36.5% |
R–Radiotherapy T–Temozolomide CR–Complete Response PR–Partial Response PD – Progressive Disease SD – Stable Disease KPS- Karnofsky performance score RS – Responders NR- Non-responders EGFR-Epidermal growth factor receptor GBM –Glioblastoma multiforme p< 0.05 =* significant Statistical test applied – Chi-square test and unpaired t-test
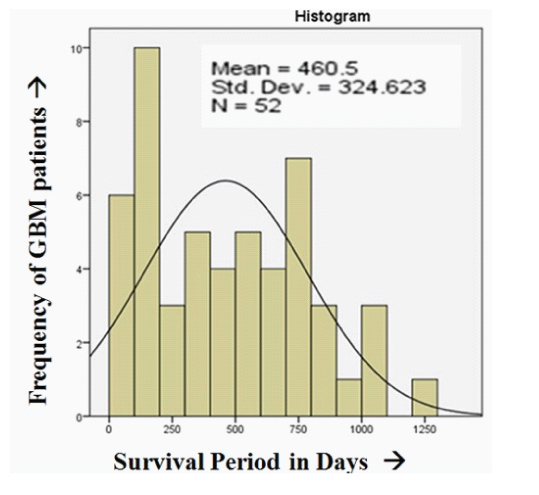
The percentage of responders in EGFR negative versus positive cases was 86.4% and 46.7% respectively. On the other hand the percentage of non-responders in EGFR negative versus positive cases was 13.6% and 53.3% respectively. This difference was significant (p-value=0.003). EGFR expression in relation to clinical characteristics of cases is detailed in [Table/Fig-4]. The mean survival among EGFR positive and negative GBM were 315.73±257.54 and 657.91±305.88 days respectively with a significant p-value (0.001) [Table/Fig-5]. The mean survival period of all 52 patients included in the study was 460.5±324.62 days [Table/Fig-6]. Our study showed insignificant relation among basic characteristics like age, sex, KPS and tumour location with EGFR protein expression.
Mean survival of GBM patients in days with respect to basic variables, treatment response and EGFR protein expression.
| Variables | Patients (n=52) | Mean Survival (Days) | p-value |
|---|
| Age (Year) |
| >40 Years | 27 | 423.85 | 0.403 |
| ≦40 Years | 25 | 500.08 |
| Sex |
| Male | 31 | 439.23 | 0.571 |
| Female | 21 | 491.9 |
| KPS |
| >50 | 28 | 482.57 | 0.601 |
| ≦50 | 24 | 434.75 |
| Tumour site |
| Frontal | 19 | 502.74 | 0.844 |
| Parietal | 14 | 461.29 |
| Temporal | 14 | 437.86 |
| Occipital | 5 | 361.2 |
| Treatment |
| R+T | 32 | 569.47 | 0.002* |
| R | 20 | 286.15 |
| Response |
| CR | 20 | 726.1 | <0.001* |
| PR | 13 | 460.15 |
| SD | 8 | 309.25 |
| PD | 11 | 88 |
| Responders |
| RS=CR+PR | 33 | 621.33 | <0.001* |
| NR=SD+PD | 19 | 181.66 |
| EGFR protein expression |
| Positive | 30 | 315.73 | <0.001* |
| Negative | 22 | 657.91 |
R-Radiotherapy, T-Temozolomide, CR-Complete Response, PR-Partial Response, SD - Stable Disease, PD - Progressive Disease, RS - Responders, NR- Non-responders, EGFR-Epidermal growth factor receptor
p< 0.05 =significant
Statistical test applied - ANOVA and unpaired t-test
Pictograph showing mean survival period.
Discussion
GBM is the most common primary WHO grade-IV tumour having very aggressive biological behaviour. Microscopically, they are characterised by pleomorphism, necrosis and endothelial proliferation with high proliferating index (MIB-1). A complete diagnostic protocol of GBM includes WHO grading, supported by IHC like GFAP, MIB-1 index and p53 expression [8].
However, along with EGFR IHC, studies on other prognostic markers like MGMT gene methylation and 1p/19q deletion should be carried out in order to guide the oncologist for institution of a rational maintenance of therapy [10,11]. Among all, EGFR gene over expression is an independent predictive marker of glioblastoma [12]. However, Isocitrate Dehydrogenase (IDH) studies are more helpful in grade-II and III astrocytoma [13].
Prognostic markers in central nervous tumour can be assessed by using IHC technique as it is simple and cost effective. It is also sensitive as well as specific and can be applied to routinely processed formalin fixed materials even if stored for long periods [14]. Considering the above advantages, anti EGFR kit was used in our study unlike what Reifenberger G et al., used in 1989 [15]. The kit in this study uses a two scale scoring system (EGFR positive and negative) unlike 4 scale scoring systems (intensity of staining 0 - no staining, 1 - light, 2 - moderate and 3 -strong) and 3 scale scoring systems (0 - no staining, 1 – light/focal, 2 – strong) described by Shinojima N et al., and Simmon ML et al., respectively [1,16].
Previous studies done by Smith JS et al., have found no association between prognosis and survival, where as our study indicates significant association between EGFR expression and OS period (mean survival among EGFR positive and negative GBM were 315.73±257.54 and 657.91±305.88 days respectively), but to establish it, further study/research is required [12]. Shinojima N et al., and Hurtt MR et al., have confined their study to supra tentorial GBM, whereas our study has included GBM in frontal, parietal, temporal and occipital sites [1,17]. Heimberger AB et al., demonstrated the prognostic effect of EGFR in cortical and/or white matter involvement (CW) and Ependymal involvement (E), whereas we have demonstrated in four locations as mentioned above [18]. Soni P et al., have observed a mean survival of 402.6 days after analysing 35 cases of GBM, whereas the mean survival period of all 52 patients included in our study was 460.5 days [19]. The response to treatment as per Soni P et al., had a CR, PR, SD and PD of 31.4%, 28.6%, 17.1% and 22.9% respectively, against CR, PR, SD and PD of 38.5%, 25%, 15.40% and 21.20% respectively in our study. Responders had better OS of 621.3 days compared to the non-responders with OS of 181.6 days due to constant follow up with patients in our study, with respect to Soni P et al., study (OS of responders and non-responders were 477.0 and 290.9 days respectively). Our study showed significant increase in OS for responders, better CR results and increased mean survival period.
Though radiotherapy was used as traditional methods and considered as a standard of care but due to its short time relief and multiple side effects there appears the necessity of some drugs that may act at molecular level for better management and prolongation of survival period. TMZ has been shown to confer better result when used as an adjuvant to RT. Our study shows better response to TMZ combined RT among EGFR negative patients (86.4%) to that of EGFR positive patients (43.3%) with a significant p-value (0.002).
Limitation
MGMT gene methylation of cases was not included in this study. TMZ therapy which is dependent on MGMT gene methylation status, can give better response.
Conclusion
GBM is a highly infiltrating tumour of the CNS and need combined approach of treatment i.e., surgery, radiation and chemotherapy. EGFR gene over expression is independent predictive markers of glioblastoma. EGFR negative patients respond better to therapy as compared to EGFR positive patients. Therefore, EGFR plays a definitive role in predicting survival of GBM patients. TMZ therapy is a better CT drug which acts at molecular level to kill the tumour cells. Its institution to the GBM patients should be guarded through EGFR IHC for better compliance. Hence, EGFR protein expression through IHC can be considered as a simple and effective ancillary technique to assess prognosis and response to therapy in GBM patients.
R–Radiotherapy T–Temozolomide CR–Complete Response PR–Partial Response PD – Progressive Disease SD – Stable Disease KPS- Karnofsky performance score RS – Responders NR- Non-responders EGFR-Epidermal growth factor receptor GBM –Glioblastoma multiforme p< 0.05 =* significant Statistical test applied – Chi-square test and unpaired t-test
R-Radiotherapy, T-Temozolomide, CR-Complete Response, PR-Partial Response, SD - Stable Disease, PD - Progressive Disease, RS - Responders, NR- Non-responders, EGFR-Epidermal growth factor receptor
*p< 0.05 =significant
Statistical test applied - ANOVA and unpaired t-test