MRKH syndrome is a Class-I congenital Mullerian anomaly (American fertility society classification 1988) [1]. MRKH syndrome affects one in 4000 females [2-4]. The major findings in MRKH syndrome are as follows: a) absence of uterus or reduced development of uterus and upper two-thirds of vagina; b) normal external genitalia; c) normally functioning ovaries; d) normal female karotype for females (46,XX) [2,3]. The MRKH syndrome had a significant influence on both fertility and psychological health of women, hence it is essential to diagnose and accurately visualize the anatomical detail to allow clinical and psychological input to patients. Surgery is necessary for restoration of normal sexual function, even reproduction may be possible if assisted reproductive techniques are performed [5]. Before surgery or assisted reproduction technique, thorough evaluation of status of uterus, ovaries and vagina are crucial for the best surgical outcome [6]. MRI is the non-invasive imaging modality of choice to detail anatomical evaluation of uterus, ovaries and vagina.
Therefore, this study was carried out to assess the MRI findings in females suspected of MRKH syndrome in a primary amenorrhea workup.
Materials and Methods
After approval from the Institutional Ethics Review Committee, a hospital based cross-sectional study was conducted. The study group comprised of 11 female patients presenting to the Departments of Obstetrics and Gynaecology and Radio-diagnosis for workup of primary amenorrhoea in a tertiary care centre from March 2016 to February 2017.
We included both outpatients and inpatients female with primary amenorrhoea and clinical suspicion of uterine anomalies. Informed consent was obtained from patients/parents/guardian before undergoing MRI scan. Medical records of all patients were retrospectively evaluated.
Initial Ultrasonography (USG) was done using Aplio-500 Machine (Toshiba Medical Systems Corporation, made in Tokyo, Japan). MRI scan were done in Siemens Avanto 1.5 Tesla B15 machine (Siemens Medical Systems, Erlangen, Germany).
All patients were subjected to MRI scan of pelvis with phased- array surface coil and obtained T1WI, T2WI and fat suppressed T2WI images in all three planes. Gradient Recalled Echo (GRE), Diffusion Weighted Images (DWI) and 3D SPACE sequences were also obtained.
Sagittal T1W images were obtained with TE: 10-12 ms,TR: 500-600 ms, slice thickness 4 mm, flip angle 1500 and FOV of 190-200. Sagittal T2WI images were obtained with TE: 90-105 ms, TR: 4800-6000 ms, slice thickness 4 mm, flip angle 1500 and FOV of 190-200. Coronal T2W images were obtained with TE: 90-105 ms, TR: 5000 -6000 ms, 256 x 256 matrix, echo train length 13-14, slice thickness 4 mm with 1 mm interslice gap, flip angle of 1500 and FOV of 240-260. Fat suppressed axial T2W images were obtained with TE: 90-100 ms, TR: 4500-5500 ms, flip angle 1500, slice thickness of 1-2 mm with no interslice gap and FOV of 200-240.
GRE images were obtained in axial planes with TE: 18-24 ms, TR: 600-660 ms, interslice gap of 1-2 mm, section thickness 3-4 mm, flip angle 28 and FOV of 200-240.
DWI was obtained in axial plane using a multi-slice spin echo planer imaging sequence. Imaging parameters were TE: 80-90 ms, TR: 2800-3200 ms, interslice gap of 1-2 mm, section thickness 3-4 mm, flip angle 900, FOV of 190-200. RF band width of 1390-1420 and matrix of 128 x 128. Diffusion probing gradients were applied in the three orthogonal directions with same strength. Diffusion weighted MR images were acquired with a diffusion weighted factor of 1000 s/mm2.
Fast (Turbo) spin echo 3D imaging technique SPACE imaging was done in coronal plane. The imaging parameters were TE: 200-250 ms, TR: 1200-1400 ms, section thickness 0.8 mm- 1 mm, flip angle 1500, FOV of 200-240. Acquisition matrix of 300 x 300.
Diagnosis of MRKH syndrome was established on the basis of non-visualization of midline uterus and absence of upper vagina. Two radiologist reaches consensus decisions for the evaluation of status of uterus, Mullerian remnants, vagina, ovaries and other associated extra-uterine anomalies like renal or lumbro-sacral spinal anomalies.
Detail evaluations of uterine remnants were done for presence, site, volumes and differentiations into layers (myometrium, functional zone and endometrium). On TIW images, the uterine remnants appears as solid elongated to ovoid structure with isointense to low signal intensities. T2W images showed the potential cavitation within the uterine buds. The cavitation appears as a target pattern consisting of a central area of T2W hyperintense signal intensity representing the endometrium surrounded by intermediate signal of junctional zone and medium to high signal intensity of muscular layer [7]. Presence of connecting/converging fibrous bands between the uterine remnants was also looked for.
The ovaries were studied for presence/absence, morphology and locations. The ovaries showed isointense to hypointense signal intensity on T1W images and mixed signal intensity on T2W images with T2 hyperintense ovarian follicles and T2W low signal intensity ovarian stroma. Extra-pelvic location of ovaries was also looked for [8]. Besides looking for ovaries in pelvic and extra-pelvic location, the relationship between the ovaries and uterine remnants was also noted. Volume calculations for the uterine remnants and ovaries were performed by measuring three orthogonal dimensions and by using the formula for the volume of an ellipsoid. The standard ellipsoid formula was length x width x height x 0.523.
The vaginal canal is better appreciable on sagittal, axial T2W and 3D SPACE imaging where vaginal canal appears as a low signal intensity structure between the urethra and urinary bladder neck anteriorly and rectum posteriorly. The T2WI hypointense muscular and fibrous tunica contrast with T2WI hyperintense mucosa and mucus within the vaginal lumen [9]. Maximum vertical length of remnant vagina was measured in sagittal T2WI or fat suppressed T2W images.
All patients were diagnosed as MRKH syndrome on basis of following features: 1) Patients with primary amenorrhea; 2) Normal development of secondary sexual characteristics; 3) Normal female gonadotropin levels including Follicular Stimulating Hormone (FSH) and Luteinizing Hormone (LH); and 4) MRI detection of an absent or vestigial uterus.
All patients’ serum FSH and LH levels were determined with radioimmunoassay methods.
Statistical Analysis
Statistical analysis was performed using SPSS programs (Statistical Package for the Social Science version 16.0 SPSS Inc., Chicago, USA). Data were presented in terms of percentage and mean.
Results
Among 11 patients of MRKH syndrome, we have found 72.7% (eight patients) had Type-I MRKH and 27.3% (three patients) had Type-II MRKH. Of 11 patients of MRKH syndrome, complete uterine and proximal vaginal agenesis was noted in nine patients (81.8%) and normally positioned small vestigial uterus in two patients (18.8%). The quantitative MRI findings in 11 patients of MRKH syndrome have been enlisted in [Table/Fig-1]. Further salient MRI findings in nine patients of MRKH syndrome with uterine and proximal vaginal agenesis have been summarized in [Table/Fig-2].
Showed the quantitative MRI findings in 11 patients of MRKH syndrome.
| Patients number | Age (yrs) | Normal positioned vestigial uterus/volume (mL) | Converging band between the uterine buds | Triangular cord sign at possible site of uterus | Right uterine bud volume (mL) | Left uterine bud volume (mL) | Right ovary volume (mL) | Left ovary volume | Vaginal length (mm) | Associated non-gynaecological anomalies | Diagnosis (Type) |
|---|
| 1. | 35 | - | + | + | 0.9 | 4 | 1.4 | 6.6 | 30.1 | No | Type -I |
| 2. | 16 | - | + | + | 0.34 | 1.4 | 7.9 | 5.1 | 21.2 | No | Type -I |
| 3. | 13 | - | - | - | 10.8 | 0.6 | 3.7 | 7.1 | 21.9 | Unilateral ectopic pelvic left Kidney | Type -II |
| 4. | 14 | - | + | + | 1.3 | 0.8 | 7.1 | 4.8 | 20.3 | No | Type -I |
| 5. | 16 | - | + | - | 0.97 | 0.7 | 5.8 | 3.7 | 20.1 | No | Type -I |
| 6. | 18 | - | + | - | 1.6 | 1.2 | 6.7 | 6.2 | 24.0 | Scoliosis of lumbar spine | Type -II |
| 7. | 17 | - | + | - | 0.11 | 0.15 | 5.5 | 8.0 | 21.5 | CRS type-V with L3 hemi-vertebra, intra-sacral meningocoele and scoliosis | Type -II |
| 8. | 22 | +/ 19.5 | - | - | - | - | 1.5 | 2.0 | 25.0 | No | Type -I |
| 9. | 15 | +/9.8 | - | - | - | - | 1.1 | 1.3 | 31.1 | No | Type -I |
| 10. | 16 | - | + | + | 2.8 | 1.5 | 7.7 | 2.25 | 37 | No | Type -I |
| 11. | 15 | - | - | - | 1.5 | 1.1 | 3.8 | 4.1 | 36.3 | No | Type -I |
Showed the salient MRI findings in nine patients of MRKH syndrome with uterine and proximal vaginal agenesis.
| MRI features | Number of findings |
|---|
| Rudimentaryuterine bud | Bilateral in nine patients, 17 located in pelvic cavity and one located in left inguinal canal. |
| Cavitation noted in one rudimentary uterine bud with more than two layers uterine differentiations. |
| T2 hypointense converging band between the rudimentary uterine buds demonstrated in seven patients. |
| Triangular cord sign in probable site of uterus demonstrated in three patients. |
| Ovary | 16 ovaries are located in pelvic cavity, one in left inguinal region and another one in right iliac fossa. |
| Vagina | Upper 2/3rd absent in all nine patients. |
The mean volume of hypoplastic normally positioned vestigial uterus in two patients was 14.6 mL±6.8 (SD). This normally located vestigial uterus showed more than one layer uterine differentiation [Table/Fig-3].
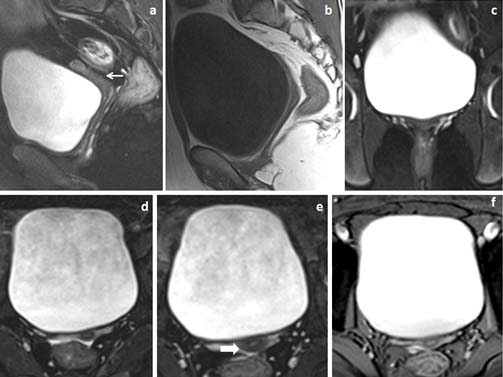
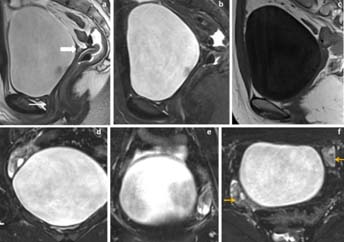
22 year’s female patient presented with primary amenorrhea. (a,b) Fat suppressed sagittal T2WI and T1W and (c) fat suppressed coronal T2W images showed marked uterine hypoplasia (white arrow in image a) with hypoplastic vagina. (d,e) Fat suppressed axial T2W and (f) axial GRE images showed differentiation of hypoplastic uterus into two layers (white block arrow in image e).
Rudimentary uterine buds were noted in nine patients. All rudimentary uteri in these nine patients were bilateral. Seventeen uterine buds (94.4%) were located laterally in the pelvic cavity in nine patients [Table/Fig-4] while in one left uterine bud (5.6%) and ovary located in left inguinal canal in one patient [Table/Fig-5]. All uterine buds had a constant caudal and medial relationship with their paired ovary [Table/Fig-4,6]. The mean volume of right uterine bud was 2.26 mL ±3.3 (SD) (range, 0.11 -10.8 mL) and left uterine bud was 1.27 mL±1.1 (SD) (range, 0.15-4 mL).
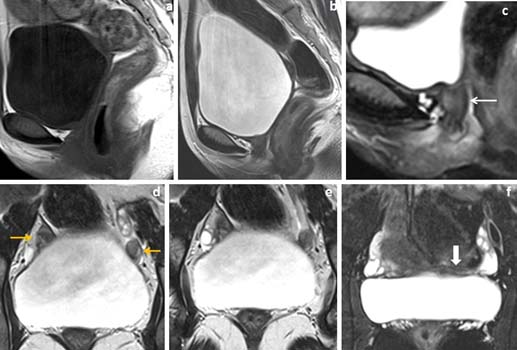
16 year’s female patient with primary infertility. (a-c) Sagittal T1WI, T2WI and fat suppressed T2W images showed absent uterus and proximal vagina (white arrow in image c). (d,e) Coronal T2W images showed T2 isointense to hypointense oval shaped rudimentary uterine buds just infero-medial to ovaries (yellow arrows in image d). (f) Fat suppressed coronal T2W image showed T2W hypointense converging band between the rudimentary uterine buds (white block arrow in image f).
35 year’s female patient with primary infertility. (a,b) Sagittal fat suppressed T2WI and T1W images showed absent uterus and proximal 2/3rd of vagina (white arrow in image a). (c) Fat suppressed coronal T2W images showed T2WI hypointense converging band (block arrow in image c). (d,e) Fat suppressed coronal and axial T2W images showed ectopic location of left ovary and rudimentary left uterine bud in left inguinal canal (yellow arrow in image d&e).
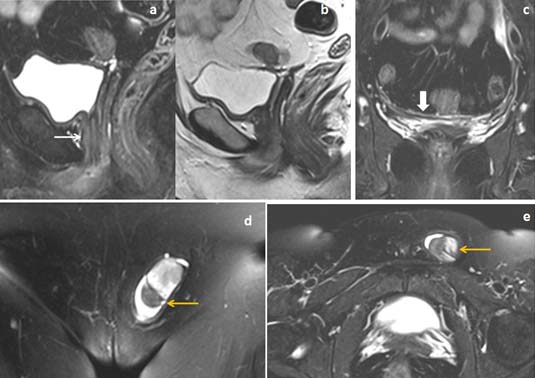
18 years female patient presented with primary amenorrhea. (a) Sagittal T2WI and (b,c) fat suppressed coronal T2W images showed agenesis of uterus and proximal 2/3rd of vagina (white arrow in image a). T2WI isointense to hypointense lesions of uterine buds located inferior and medial to the ovaries (yellow arrows in image b) and horizontally oriented converging T2WI hypointense band noted between the rudimentary uterine buds (block arrow in image c). (d,e) Fat suppressed axial T2W images showed normal follicular activities in bilateral ovaries (red arrow in image d).
These seventeen rudimentary uterine buds (94.4%) did not shows differentiations into more than one layer while only one uterine bud (5.6%) showed differentiation into more than two layers [Table/Fig-7].
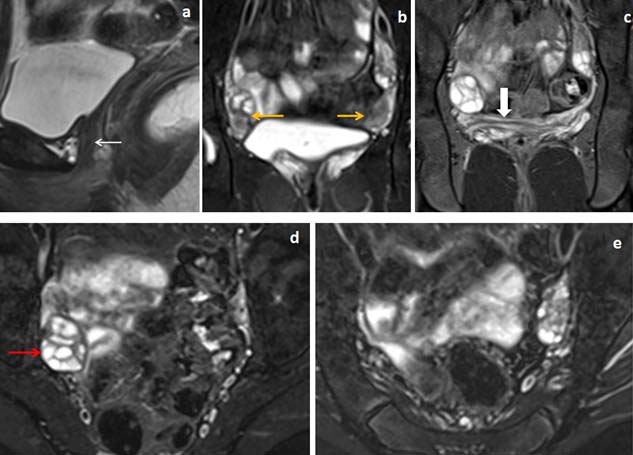
13 years female presented with primary amenorrhea and lower abdominal pain. (a,b) Fat suppressed sagittal and coronal T2WI and (c) sagittal T2W images showed uterine and proximal vaginal agenesis (white arrow in image a) with midline ectopic location of left Kidney in supra-pubic region. Right rudimentary uterine bud showed differentiations into three layers (yellow arrow in image b) with small rudimentary left sided uterine remnant located below left ovary (white block arrow in image c). (d,e) Axial T1WI and fat suppressed T2WI images showed T1WI hyperintense blood product within the functioning endometrium of right rudimentary uterine bud (yellow arrow in image d and e).
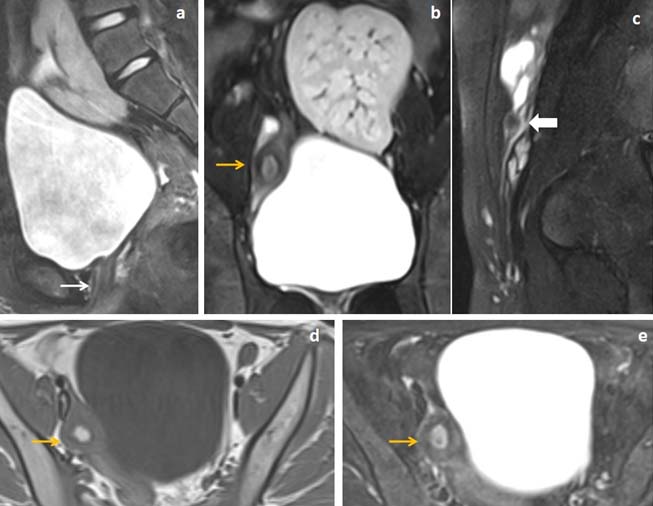
The T2WI hypointense converging band was noted in between the rudimentary uterine buds in seven patients (77.8%) out of nine patients [Table/Fig-5,6,8]. The midline triangular soft tissue were noted in four patients (44.4%) in probable site of uterus in retro-vesical location [Table/Fig-8,9].
16 years female presented with primary amenorrhea. (a,b,c) Fat suppressed sagittal T2WI and sagittal T1W images showed uterine and upper vaginal agenesis with T2 mixed signal intensity and T1 isointense triangular cord like lesion in probable site of uterus in retro-vesical location (black arrow in image b and white arrow in image c). (d,e) Fat suppressed coronal and axial T2W images (d and e) showed normally functioning bilateral ovaries with follicles with T2 hypointense converging band between the uterine buds (yellow arrow in image d) and with T2 isointense bilateral uterine buds (white block arrows in image e).
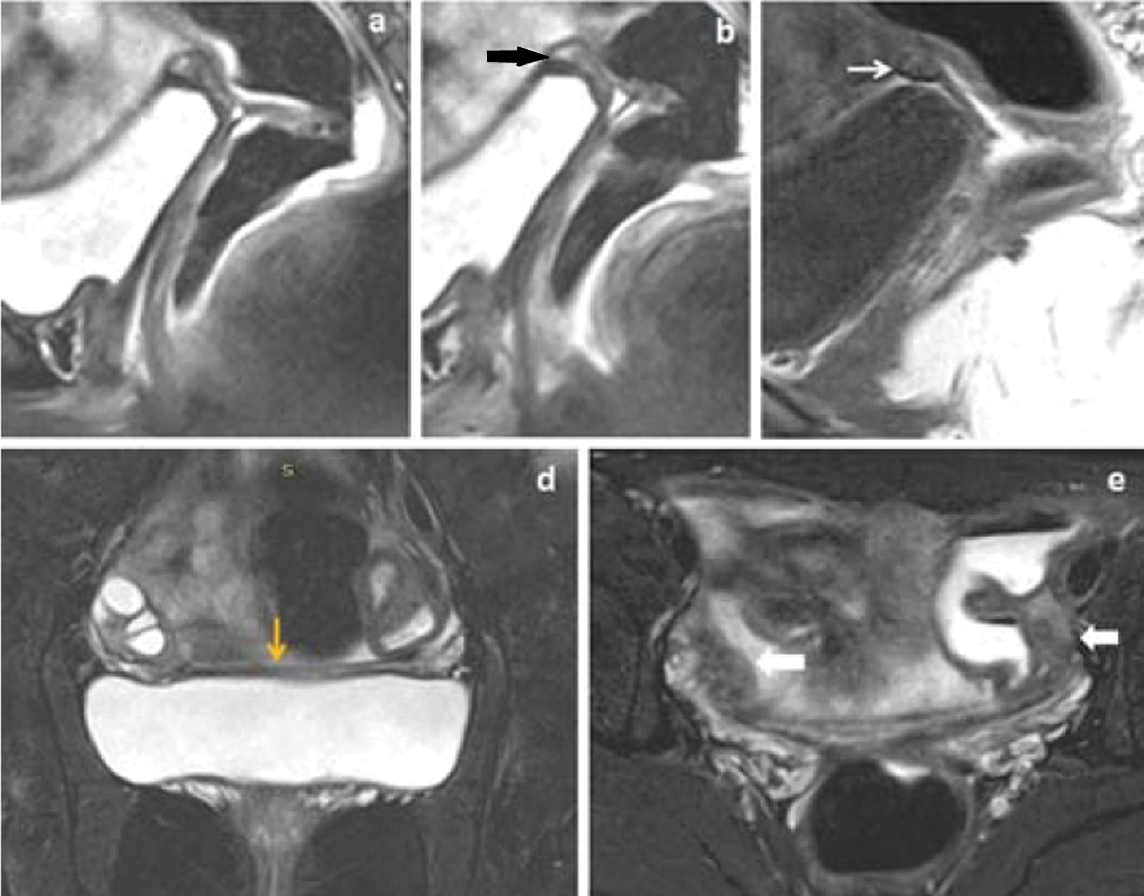
14 years female presented with primary amenorrhea. (a-c) Sagittal T2WI, fat suppressed T2WI and T1W images showed agenesis of uterus and upper vagina (white arrow in image a) with triangular cord like lesion in probable site of uterus in retro-vesical location (white block arrow in image a). (d-f) Fat suppressed coronal and axial T2W images showed normally functioning bilateral ovaries with active follicles (yellow arrows in image f).
Bilateral ovaries and fallopian tubes were present in all 11 patients. Twenty ovaries (90.9%) were located in lateral side of pelvic cavity, one ovary (4.5%) located in left inguinal canal [Table/Fig-5] and another one (4.5%) located in right iliac fossa. The mean volume of right ovary was 4.75 mL±2.6 (SD) (range, 1.1-7.9 mL) and left ovary was 4.65 mL±2.2 (SD) (range, 1.3-8 mL).
Proximal 2/3rd of vagina was not developed in nine patients (81.8%) [Table/Fig-4,5 and 6,8] while two patients (18.2%) had development of whole vaginal length with hypoplastic vaginal canal [Table/Fig-3]. The mean vaginal length in 11 patients was 26.2 mm±6.34 (SD) (range, 20.1-37 mm) Associated renal anomaly in the form of unilateral renal ectopia noted in one patient (9%) of MRKH [Table/Fig-7]. Another one patient (9%) had lumbro-sacral anomalies as coccygeal agenesis (Type-V Caudal regression syndrome), intra-sacral meningocoele and L3 hemi-vertebra. Two patients (18.2%) of MRKH had scoliosis of lumbar spine.
Discussion
The MRKH syndrome was first described by Mayer in 1829 and by Rokitansky in 1838 while Hauser and Schreiner in 1961 described the distinguishing features of MRKH syndrome from androgen insensitivity syndrome [10]. Associations of MRKH syndrome are GRES (Genital, renal and ear syndrome) and MURCS (Mullerian, Renal and Cervical Somite dysplasia) [10,11].
There are two forms of MRKH syndrome: a) typical form (Type-I) is characterized by only congenital absence of uterus and upper vagina with normal appearing ovaries and fallopian tubes; and b) atypical form (Type-II) includes Mullerian anomalies associated with non-gynaecological anomalies of urological, skeletal, vertebral or cardiac systems [12].
Urological abnormalities associated with Type-II MRKH syndrome are renal ectopia, horse shoe kidney and rarely renal agenesis. Varying degrees of musculoskeletal anomalies have been noted ranging from vertebral segmentation anomalies, scoliosis to abnormalities of radius, carpals, phalanges and femoral capital epiphyses. Ovarian cancers [13,14] and cardiac malformations [15,16] have been reported with Type-II MRKH syndrome. Differentiations between MRKH syndrome and androgen insensitivity syndrome are essential for treatment planning of such patients.
The MRKH syndrome arises due to arrested development of paramesonephric ducts seven weeks after fertilization [17]. The Mullerian (paramesonephric) ducts form the uterus, cervix, upper 2/3rd of vagina and fallopian tubes [18]. If the endometrial layer in uterine is functional, the patients of MRKH syndrome may present with primary amenorrhea and cyclical abdominal pain due to cryptomenorrhea and haematometra [19].
Clinical presentation is characterized by primary amenorrhea and normal development of secondary sexual characteristics due to normal development of ovaries and normal ovarian functions. The levels of FSH and LH are normal with no sign of androgen excess which can be differentiate from androgen insensitive syndrome [20].
USG should be the first investigation in evaluating suspected MRKH syndrome in primary amenorrhea patients. While USG may not always detect the uterine buds or ovaries even in ectopic location, it can falsely detect rectovesical quadrangular structure as hypoplastic uterus. Information about rudimentary buds is essential before surgical treatment outcome [21].
The MRKH syndrome should be differentiated from androgen insensitivity syndrome, and isolated vaginal hypoplasia or atresia. In androgen insensitive syndrome, end organ resistance to androgen resulting in virilisation of external genitalia resulting in female phenotype of baby with development of female secondary sexual characteristics and genotypically male (46XY) with undesecended testes which MRI can differentiate from MRKH syndrome [22]. MRI detect absence of uterus and ovaries with presence of rudimentary ectopic testis in androgen insensitive syndrome [23]. The rare disorder called WNT4 syndrome (Biason-Lauber syndrome) affect genetically and phenotypically female patient caused by mutation in WNT4 gene resulting in partial virilisation of embryonic ovaries with secretion of both estrogen and androgen resulting high androgen level causing Mullerian inhibition and non-formation of uterus and proximal vagina and development of female secondary sexual characteristics [7]. Isolated vaginal hypoplasia or atresia may be seen in syndromes like Mc Kusick-Kaufmann syndrome, Winter syndrome and Fraser syndrome [24]. In these syndromes, patients are genotypically female with normal development of uterus and ovaries with variable defect of vagina which can be detected with help of MRI.
Thus MRI in MRKH syndrome can not only be used as a non-invasive technique alternative to diagnostic laparoscopy but also to differentiate this entity from other possible differentials [3]. Pompili G et al., assessed the accuracy of MRI findings in MRKH syndrome with respect to diagnostic laparoscopy [3]. Though our study does not aim to assess absolute accuracy of MRI, our results revealed good correlation between the MRI and laparocopy findings in keeping with the study by Pomili G et al., [3]. Yoo R-E et al., assessed the detailed characteristics of MRI findings in uterine remnants of MRKH syndrome [24]. MRI findings in our study as well nicely delineated the uterine anomalies, picked up uterine remnants in majority of the cases and identified accurately normal or ectopic location of uterine remnants and ovaries. And our study findings were compared with previous published literatures in last two decades [Table/Fig-10].
Literature review in MRI findings of MRKH syndrome associated with uterine and proximal vaginal agenesis in last two decades.
| Series/ year | Number of cases | Age / mean age (yrs) | Location of Rudimentary Uterine bud | T2 hypointense converging band in between the uterine bud (n) | Triangular cord sign at possible site of uterus(n) | Endometrial differentiations in uterine bud | Location of ovaries (in numbers) | Identified lower 1/3rd vagina(in numbers of patient) | Associated non-gynecological anomalies |
|---|
| Reinhold C et al., [4] | 12 | - | Uterine agenesis -9,unicornuate hypoplastic uterus-1, atresia of lower uterine segment-1,small fibrous remnant-1 | - | - | - | Pelvic cavity-24.One left ovary shows endometrioma. | Complete vaginal agenesis-5,Agenesis of proximal 2/3rd-3,Fibrous remnant -2,Agenesis of proximal 1/3rd-2 | - |
| Pompili G et al., [3] | 56 | 20.9 | Uterine buds bilateral -68 (60.7%)Unilateral-20 (17.8%)Not seen-24 (21.4%) | - | - | - | Bilateral -108Unilateral -4,Pelvic-96(85.7%),Extra-pelvic-16(14.3%) | Lower 1/3rd-17 patients,Complete agenesis-38 patients | Renal-9 |
| Giusti S et al., [2] | 1 | 15 | Hypoplastic blind ended uterus located right iliac fossa | - | - | More than 2 layers | Pelvic cavity-2 | 1 | - |
| Yoo R-E et al., [24] | 15 | 23.7 | Pelvic cavity -30 | 15 (100%) | 13 (86.7%) Midline -11, para-median -2) | only 1 layer- 28 uterine buds,more than 2 layers-2 | Pelvis-30(100%) | 14 (93%) | Renal=2(13.3%)Vertebral=4 (26.7%) |
| Kara T et al., [29] | 16 | 19.4 | Uterine aplasia -5(31.3%), Uterine hypoplasia-11(68.8%) | - | - | - | Pelvic cavity-21Not detected -10, agenesis -1 | 16 (100%) | Renal=4Vertebral=2 |
| Hall-Craggs MA et al ., [30] | 66 | 21.1 | 61 patients (92%) total of 115 uterine buds detected.Bilateral-108(82%),unilateral-7(11%),ecmiddleic in inguinal canals-2 | 4 (6%) | - | 24 uterine buds(21%) of 115 showed endometrial differentiation, where 15 (13%) buds showed more than 2 layer and 9(8%) showed 3 layers. | Detected -108,Pelvic cavity-54Ectopic -54 (bilateral-26 and unilateral-27) | 44 (66.7%) | Renal-13 |
| Present study | 9 | 17.8 | 17 located in pelvic cavity and 1 located in left inguinal canal. | 7 (77.8%)patients | 3 (33.3%)patients | 17 uterine buds shows only 1 layer and 1 uterine bud shows more than 2 layers | 16 (88.9%) ovaries are located in pelvic cavity, one in left inguinal canal and another one in right iliac fossa. | 9 (100%) | Ectopic kidney -1, scoliosis of lumbar spine-2, CRS-V with intrasacral meningocoele and hemi-vertebra-1, |
The aim of management of MRKH syndrome patients are to create a functional neo-vagina with adequate length and diameter to accommodate sexual intercourse [25,26]. The management consists of creation of a new vaginal canal either surgically or non-surgically with help of Frank’s dilation method and second surgical technique with various vaginoplasty [27]. Diagnostic laparoscopy or celioscopy is an invasive and expensive diagnostic method in assessment of patients with MRKH syndrome and requires patient hospitalisation. Celioscopy is now a days mainly reserved for patients with indeterminate MRI scans or for women in whom interventional therapy is likely to be undertaken (for example construction of a neo-vagina) [28]. However, MRI by superior soft tissue contrasts resolution, non-invasiveness, multi-planar capability has come up as an essential investigation for detailed preoperative evaluation of patients with suspected MRKH syndrome. The bulk of diagnostic information obtained from MRI in such patients has made it a crucial investigation of choice unlike earlier times, when MRI was indicated following inconclusive/indeterminate USG. Multiplanar capability of MRI can be exploited effectively to detect and characterize various anomalies in such patients for example the uterine aplasia is best detected on sagittal plane, whereas the vaginal atresia can be better diagnosed on axial plane [28]. These surgical or non-surgical management are more difficult in Type-II MRKH patients. However, relative good outcome in the form attainment of sexual intercourse were noted in patients with uterine hypoplasia than Type-I MRKH. Hence, preoperative MRI evaluation of genital and other associated anomalies help in prognosticating the patients with uterine hypoplasia or MRKH.
Limitation
Only 11 patients of MRKH syndrome were included in the sample to assess the various MRI findings. We look forward to do a prospective study in future including a larger sample size with longer duration.
Conclusion
MRKH is a congenital malformation with variable degree uterovaginal agenesis with functional ovaries. MRKH alert to look for urinary, skeletal or cardiac anomalies. Psychological consideration is needed in MRKH patients and treated either creation of neovagina or attainment of reproduction with newer assisted reproduction techniques.
Those MRKH patients who have uterovaginal agenesis with rudimentary uterine buds and those patients with vestigial uterus had different prognosis or outcome even though also in Type-I and Type-II MRKH, where Type-II had poor prognosis.
Adequate MRI assessment in these MRKH patients aid in counselling the patients/parents/guardian in a better way preoperatively, hence MRI can emerge as a major tool in diagnosing as well as prognosticating outcome in such MRKH patients.