General anaesthesia with FGF at rates less than 2 L/min is associated with several advantages including improved pulmonary environment, mucociliary clearance and body temperature maintenance. In addition, it results in anaesthesia cost reduction of almost 75% [1-3] and has significant environmental benefits in terms of reduction in theatre pollution with anaesthetic gases [4].
In this study, we attempted to study the intra-operative gas changes with the use of N2O-free anaesthesia with medical air and oxygen at low FGF rates of 300 mL/min each in a two-hour period. Our primary objective was to record the changes in anaesthetic agent and oxygen concentration and the least FiO2 levels achieved, with the proposition that any value of FiO2 below 0.3 would render this technique unsafe for clinical use. The analgesic requirements and cost difference and complication rates compared to low flow anaesthesia by using N2O and oxygen in similar flows were also analysed.
Materials and Methods
This was a prospective observational study that was conducted between March 2015 and June 2016 at the Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, after the approval of the Institutional Ethical Committee. All patients provided a written informed consent for their inclusion in the study. As this study was planned as a feasibility study, a sample size of 40 was planned in each group. All patients were blinded to the procedure. The first author analysed the data and was blinded to the anaesthetic technique.
Patient selection: Patients in the age group of 18 to 60 years, with American Society of Anaesthesiologist, Physical Status (ASA PS) of 1 or 2 undergoing surgical operations requiring general anaesthesia with controlled ventilation and lasting more than two hours, were included in the study. Patients with chronic respiratory diseases and those undergoing cardiothoracic and neurological operations or surgical operations with significant haemodynamic changes, were excluded from the study. Patients receiving any other form of analgesia were also excluded from the study. Patients that satisfied the inclusion criteria were randomized into one of the two groups, Group O (without nitrous oxide) and Group N (with nitrous oxide).
Anaesthesia: All patients were premedicated with morphine 0.1mg/kg intramuscularly and diazepam 5 mg orally prior to surgery. Intravenous access was secured and monitors were connected. All patients were pre oxygenated for three minutes with 100% oxygen and induced with 4-5 mg/kg of thiopentone and 2 μg/kg of fentanyl. Intubation was facilitated with 0.1 mg/kg of vecuronium.
Study group: After intravenous induction, anaesthesia was maintained in patients in the study group (Group O) according to standard institute protocol with 3 L/minute each of medical air and oxygen for three minutes, along with sevoflurane to achieve a Minimum Alveolar Concentration (MAC) of 1-1.2. After three minutes, the trachea was intubated, during which time, the flows were stopped. After intubation, a leak free circle system was connected and the gases were continued at the same flow rates for a further seven minutes. The FGF was reduced to low flow with 300 mL/min each of medical air and O2 and the dial setting of sevoflurane was maintained to achieve a Minimum Alveolar Concentration (MAC) between 1-1.2 for the remaining duration of the surgery.
Anaesthesia in patients of Group N was maintained with oxygen and N2O at flow rates of 3 L/min each and sevoflurane by using the same technique as described above, after intravenous induction of anaesthesia. At the end of 10 minutes, the flow rates of both oxygen and N2O were reduced to low flow with each at 300 mL/min. The dial setting of sevoflurane vaporiser was adjusted to maintain MAC between 1-1.2. The main purpose of this group was for comparing the cost and analgesic requirements. The dial setting in the sevoflurane vaporiser was increased in increments of 0.5 in the event of an increase in the heart rate and blood pressure of the patient by more than 20% of baseline. Sevoflurane was reduced similarly if the Heart Rate (HR) and Systolic Blood Pressure (SBP) decreased more than 20% of baseline. In the event of persistent elevation of blood pressure or heart rate, fentanyl was administered intravenously in boluses of 20 μg. This was also based on the decision of the attending anaesthesiologist.
All patients were ventilated to achieve an ETCO2 of 32-35 mmHg. The anaesthesia machine used was capable of delivering low flows (Aestiva/5 7100, Software Revision 1.X, GE Healthcare, Madison, WI). Amsorb® (Calcium Hydroxide and calcium chloride) was used as the CO2 absorbent.
Data collection: The data was collected by using a multi-parameter monitor with facility for gas monitoring (Datex-Ohmeda S/5TM Monitor, GE Healthcare). The accuracy of gas monitoring was 0.2 with a rise time of <400 msec. The parameters monitored were ECG, HR, SpO2, SBP, DBP, Fraction of Inspired Oxygen (FiO2), End Tidal Oxygen (ETO2), Fraction of Inspired Nitrous Oxide (FiN2O), End Tidal Nitrous Oxide (ETN2O), Fraction of Inspired Sevoflurane (FiSevo), End Tidal Sevoflurane (ETSevo), Fraction of Inspired Carbon Dioxide (FiCO2), End Tidal Carbon Dioxide (ETCO2), Minimum Alveolar Concentration (MAC) of sevoflurane and the dial setting of vaporiser. Data was recorded at five minute intervals.
Rescue measure: A drop in FiO2 below 0.3 was fixed as the trigger to provide increased flows to administer a higher FiO2. In case of a circuit disconnection intra-operatively, flows would be shut off and restarted following reconnection at the same flow rates.
The complications assessed were postoperative nausea and vomiting, intra-operative awareness (assessed by using Brice questionnaire), delirium and postoperative myocardial infarction.
Outcome measures: The changes in the gas composition, specifically FiO2, was analysed to identify the safety of using medical air and oxygen in low flows over a period of two hours. Even a single recording of FiO2 less than 0.3 would deem the technique as unsafe for clinical practice. The mean maximum and minimum values of FiO2, ETO2, FiSevo, ETSevo, MAC and ETCO2 were measured in both the groups. Additionally, FiN2O and ETN2O were measured in group N. SPSS software version 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp) was used to generate data and figures.
Statistical Analysis
The cost of sevoflurane was computed, based on the consumption of total liquid sevoflurane as demonstrated by the dial setting and FGF. The average cost incurred was compared between both the groups. Fentanyl requirements between the two groups were compared by using unpaired t-test.
Results
Of the 80 patients satisfying the inclusion criteria, 10 were excluded for intra-operative haemodynamic instability (three patients), circuit disconnections and leaks (four patients) and prolonged time taken to intubate (three patients). Eventually, 35 patients were included in each group for analysis.
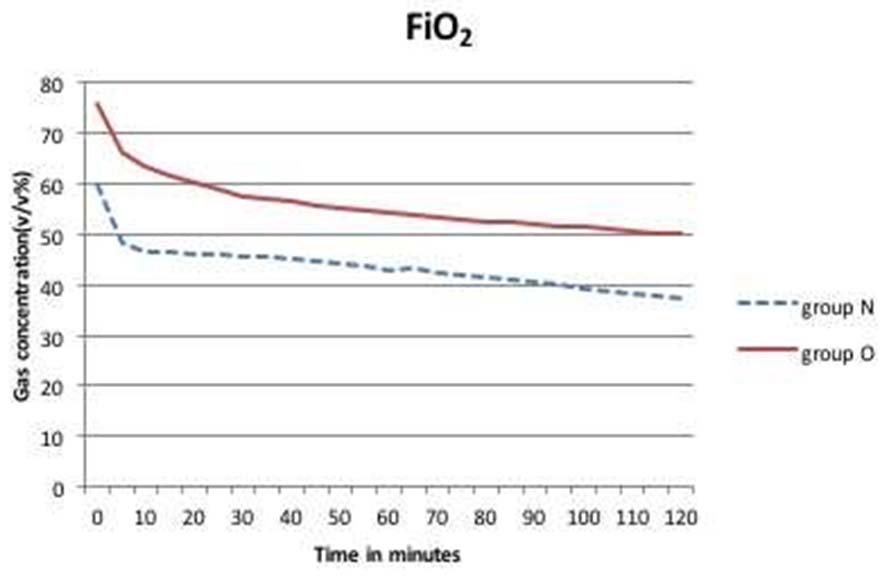
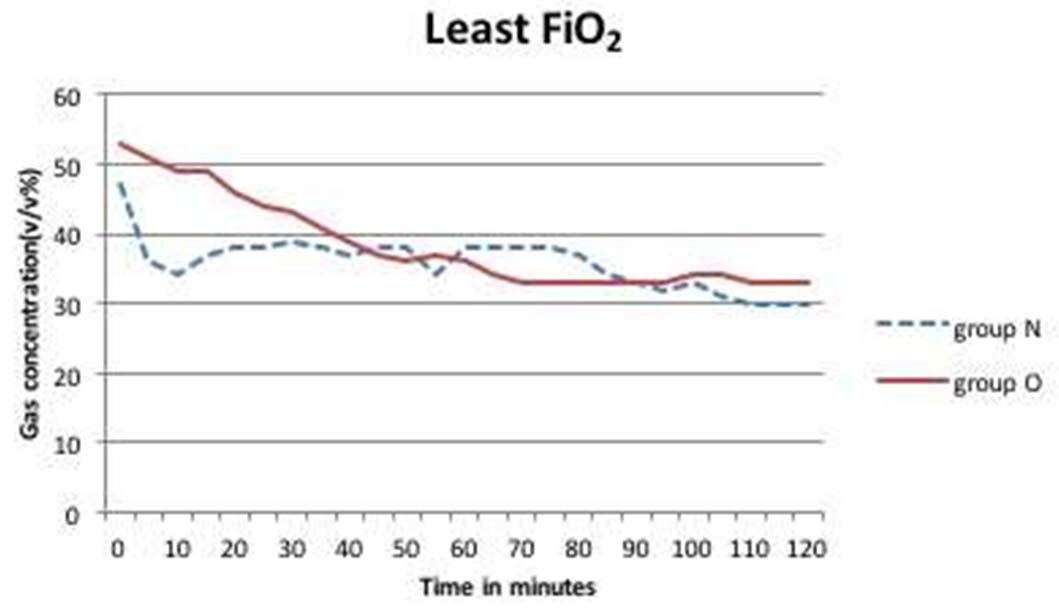
The patient demographics in both the groups were comparable [Table/Fig-1]. The mean inspired oxygen concentration in the first hour in Group O and N were 54.4±18 v/v% and 43.1±6.2 v/v%, respectively [Table/Fig-2]. At the end of second hour mean FiO2was 50.2±16.8 v/v% in Group O and 37.5±7.4 v/v% in group N. The least recorded FiO2 value was 33 v/v% in Group O and 30 v/v% in Group N [Table/Fig-3]. There was no value recorded below 0.3 in the 140 study hours recorded.
Comparison of demographic data between the groups.
| Parameters | Group N | Group O | p-value* |
|---|
| Mean age (years) | 45 | 45 | 0.9 |
| Mean weight (Kg) | 56.1 | 58 | 0.77 |
| Male:female | 4:3 | 5:2 | 0.31 |
| ASA 1:ASA 2 | 22:13 | 16:19 | 0.62 |
* Student’s t-test
Mean FiO2 concentrations in Group O and Group N over two hours.
Least FiO2 levels attained in Group O and Group N over a two hour period.
Comparison of the trends in mean inspired and end tidal oxygen concentration revealed ETO2 to be greater than FiO2 for the initial five minutes. Subsequently, it dropped to a level lower than FiO2. The difference between FiO2 and ETO2 stabilized after a period of 20 minutes to a mean value of 2.8 v/v% (2.4-3 v.v%) for the remaining duration of observed anaesthesia.
In the control group, the uptake of N2O declined following an initial period of higher uptake. After around 20 minutes on low flows, the N2O concentration in inspired gas steadily increased as its uptake came down.
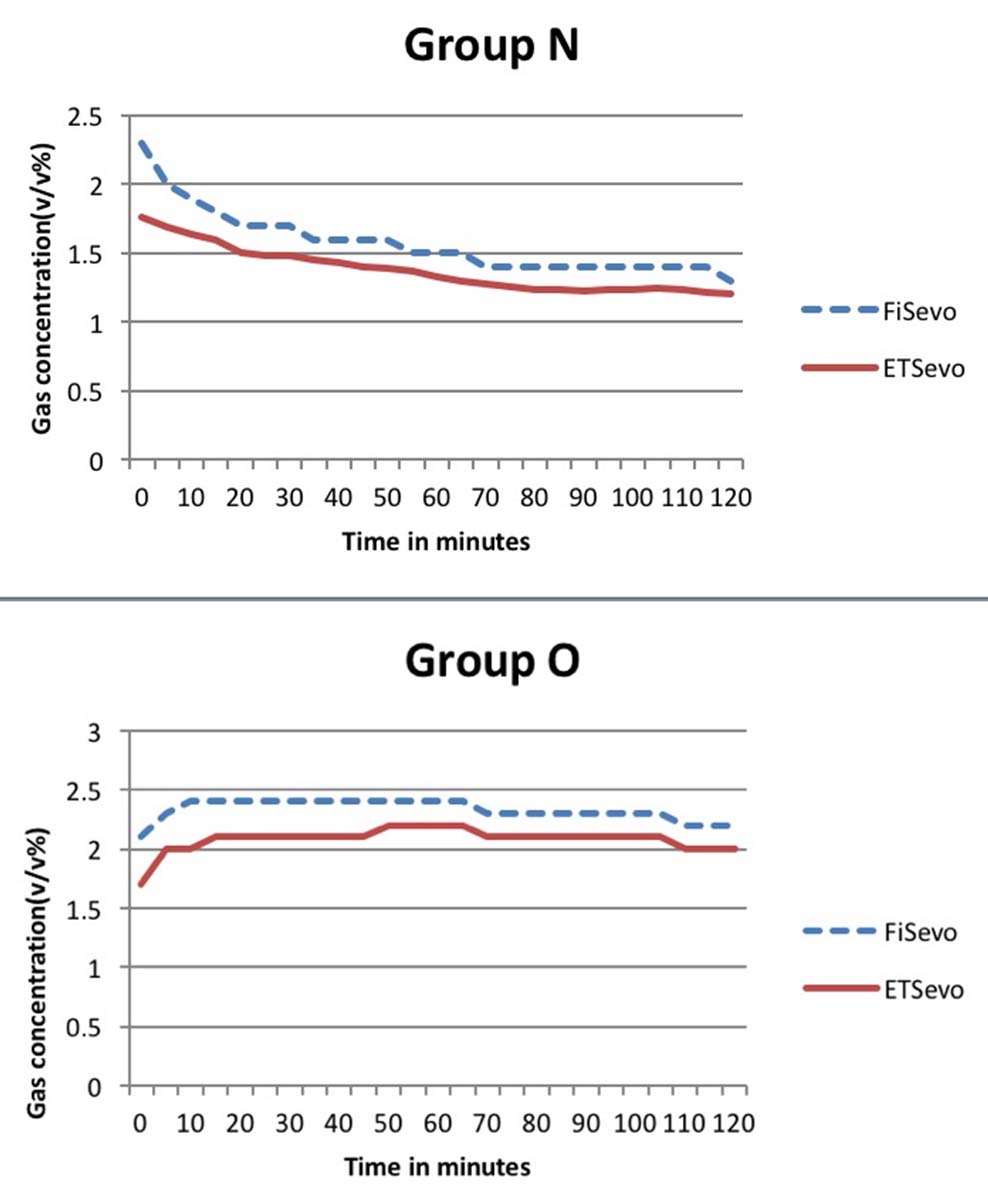
Dial settings for sevoflurane vaporiser in Group N was 4% during the initial 10 minutes in high flow and subsequently came down to 2% in the first half hour, 3% during the following one hour and 2% till the end of two hours. In Group O, Dial setting was kept at 5% in high flows and subsequently reduced to 4% in first half hour in low flow, 5% in the subsequent hour and 4% till the end of two hours.
The inspired sevoflurane concentration was around 2±0.44 v/v% for the first 10 minutes in high flows. The concentration gradually reduced over the first hour to reach a level around 1.4±0.48 v/v% which was constant for the next one hour in Group N. In Group O, the mean inspired sevoflurane concentration in the initial few minutes was 2.1±0.60 v/v% which settled to a constant value of 2.3±0.60 v/v% for the rest of the period [Table/Fig-4].
Inspired and end tidal sevoflurane concentration in Group N and O.
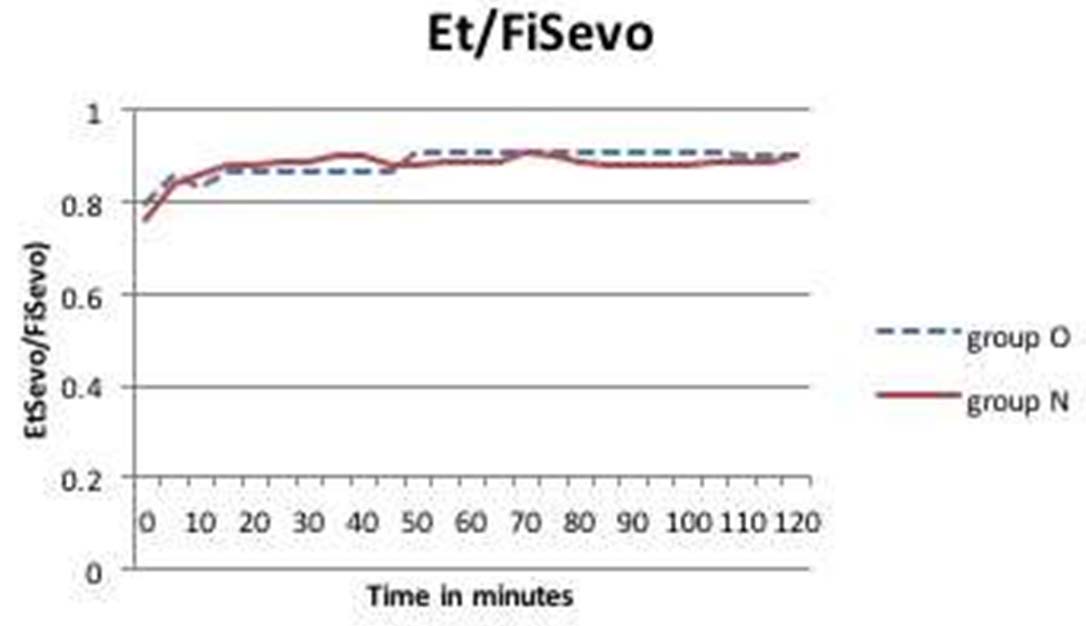
In our study the difference between inspired and end tidal sevoflurane concentration was around 0.2-0.4 v/v% and 0.1-0.2 v/v% in the study and control groups, respectively. In the study group (Group O), the ratio of end tidal to inspired sevoflurane concentration reached a value of 0.8 in the first 15 minutes and as dial setting of sevoflurane was increased one hour after induction, the ratio became 0.9 within five minutes and remained constant after that. In Group N, the ratio of end tidal to inspired sevoflurane reached equilibrium of 0.9 in 15 minutes and remained constant throughout the study period [Table/Fig-5].
Ratio of End tidal vs. Inspired concentration of sevoflurane in Group O and N over a two hour period.
The MAC was maintained between 1-1.2 throughout the two hour period in both the groups. The end tidal carbon dioxide concentration was maintained between 33-34 mmHg in both the groups.
In the study group, the mean heart rate was 72-94 beats/min. The mean systolic and diastolic blood pressure was in the range of 107-120 mmHg and 55-70 mmHg, respectively. There were no significant fluctuations. Similarly, in the control group, the mean heart rate, systolic and diastolic blood pressure was 73-92/min, 107-120 mmHg and 62-76 mmHg, respectively [Table/Fig-6].
Haemodynamic variables in both the groups.
| Parameter | Group O | Group N |
|---|
| Mean Systolic Blood Pressure (mmHg) | 107-120 | 107-120 |
| Mean Diastolic Blood Pressure (mmHg) | 55-70 | 62-76 |
| Mean Heart Rate (beats/min) | 72-94 | 73-92 |
The mean total fentanyl requirements in the study (Group O) and control groups (Group N) were 151.85 μg and 124.85 μg, respectively. This difference between the two groups was statistically significant (p-value=0.0004).
None of the patients, followed up in this study, reported any awareness during surgery or postoperative nausea and vomiting. There were no incidences of delirium or postoperative myocardial infarction observed.
The total cost incurred for anaesthesia per patient in the study and the control groups was INR 688.2 and 424.6, respectively [Table/Fig-7] with a 62% increase in sevoflurane utilization in Group O.
Comparison of the costs incurred between Group N and Group O in low flow anaesthesia.
| Agent | Flows | Group N | Group O |
|---|
| Volume(ml) | cost(INR) | Volume(ml) | Cost(INR) |
|---|
| Sevoflurane*(INR 19.4/ml) | High flows @ 6 L/minLow flow @600 ml/min | 9.7 ml9.2 ml | 188178.6 | 16.3 ml16.3 ml | 316.22316.2 |
| Nitrous oxide**(INR 190/1000 l) | High flow @3 L/minLow flow @ 300 ml/min for 110 min | 30 000 ml33 000 ml | 5.76.27 | - | |
| Oxygen**(INR 19/1000 l) | High flow @3 L/minLow flow @300 ml/min | 30 000 ml33 000 ml | 0.570.63 | 30 000 ml33 000 ml | 0.570.63 |
| Fentanyl(INR 36/100 μg) | | | INR 44.9 | | INR 54.6 |
| Total cost*** | | | INR 424.6 | | INR 688.2 |
*1ml of sevoflurane gives 184 ml of saturated vapour, according to the formula, Fluid (VA) mL = (specific wt) × Avagadro’s constant × (273 + temp) ÷ (mol.wt × 273),
**The formula for the calculation of amount of volatile agent utilised is given by Fluid vol.agent= (mean FGF × mean agent × duration) ÷ (sat.gas vol × 100)
***The cost of amsorb is not included in the analysis as there is no difference in the fresh gas flows.
Discussion
The safety of using low flow anaesthesia is well established and its benefits in terms of cost savings, operation room and personnel safety due to reduced wastage of anaesthetic gases, are universally accepted [1,2,12-15]. However, the use of nitrous oxide is being increasingly questioned in current practice. We performed this study to assess the safety of using oxygen in combination with medical air. We monitored intra-operative gas composition while using sevoflurane as the anaesthetic agent.
In this study, we set a fixed flow rate of 600 mL/min of FGF with the study group receiving 300 mL/min of O2 and medical air and the control group receiving 300 mL/min of N2O and O2 each following an initial 10-minute period of high flows (three minutes before and seven minutes following intubation). The haemodynamic parameters, blood pressure and heart rate were stable throughout in both the groups on low flows without any fluctuations.
In a similar study using different proportions of N2O and O2, Virtue RW et al., monitored gas composition and reported that in the initial hour on low flows, the FiO2 never dropped to less than 30%. In the group receiving N2O and O2 in a 50:50 ratio, Virtue RW et al recorded a FiO2 of 24% as the least recorded value at the end of two hours [16]. In our study too, there were no recordings of FIO2less than 30% and the least recorded value was 33 v/v%. During the initial five minutes in the control group, ETO2 is higher than FiO2 as there is rapid uptake of N2O. The initial increase in ETO2 more than that of inspired O2 is attributable to the high initial alveolar uptake of nitrous oxide. Over time, as the nitrous oxide uptake decreases exponentially, the difference between the inspired and expired oxygen concentration stabilizes as the uptake of oxygen became constant.
Lin CY et al., found that the oxygen consumption is constant during general anaesthesia at the rate of 250-300 mL/min [17]. Although we did not directly measure O2 consumption, we calculated the difference between the inspired and expired concentration of oxygen and found that after an initial period where the difference was high, indicating a higher uptake, it stabilized at 2.4-3 v/v% indicating a constant uptake of oxygen in both the groups. Bengston JP et al., and Raymond JA et al., have also reported similar observations. They have additionally found that the difference between FiO2 and ETO2 increases as the fresh gas flow decreases, a trend that is corroborated by our data [18,19].
The volume of gas in the circuit must be maintained to prevent the delivery of a hypoxic mixture. We have maintained a FGF of 600 mL/min in our study and a ratio of 50% oxygen was maintained in the study group. This is recommended for attaining FiO2 of 0.3 in low flow anaesthesia as flow reduction may result in increased rebreathing of oxygen-depleted gas. In the control group, we observed a decline in the uptake of N2O following an initial period of higher uptake. After around 20 minutes on low flows, the N2O concentration in inspired gas steadily increased as its uptake came down. However, the minute-by-minute O2 consumption remained constant. This highlights the potential for dangerous accumulation of N2O in the circuit as oxygen concentration falls during prolonged anaesthesia in low flows. Such a risk is non-existent in the study group that received the oxygen-medical air mixture.
Sevoflurane is one of the newer inhalational agents that were initially discouraged from use in low flows for more than two MAC hours due to the risk of compound A formation. Sevoflurane degrades to compound A, which is found to be nephrotoxic in rats and the formation of compound A is more likely in low flow conditions. Therefore, it is now used frequently in low flows with alkali free CO2 absorbents like Amsorb [20]. The rapid uptake and elimination of sevoflurane also favours its use in low flows. In this study we maintained a MAC between 1 to 1.2 and adjusted dial settings accordingly to achieve the same. The haemodynamic parameters also guided the titration of dial settings. The depth of anaesthesia was found to be adequate in all the patients in our study.
Several theories have been proposed to explain the uptake and distribution of gases during low and minimal flow anaesthesia. Two of the generally accepted ones are the Lowe’s theory (that states that uptake is inversely proportional to square root of time) and the Lin’s theory (that states that there is an initial wash-in period which equilibrates the entire circuit and the Functional Residual Capacity (FRC) of the patient with the anaesthetic gases following which there is only a constant uptake of gases throughout the duration of anaesthesia) [17]. Several studies have confirmed that the uptake of anaesthetic agents remains constant following an initial period of high uptake [12,21]. We were guided by Lin’s theory and included an initial 10 minutes of high flows followed by a reduction in gas flow rates for the rest of the anaesthesia. This reduces the need for frequent changes in dial settings of the vaporiser and avoids complex calculations. Gorsky BH et al., pointed out that there is only a slight change in the uptake of anaesthetic agent during general anaesthesia [12]. In our study the difference between inspired and end tidal sevoflurane concentration was around 0.2-0.4 v/v% and 0.1-0.2 v/v% in the study and control groups, respectively. The ratio of expired to inspired concentration of sevoflurane reached a constant of 0.8 and 0.9 in the study and control groups, respectively, at 15 minutes. We also observed that an increase in dial setting after the first 30 minutes resulted in achieving a new equilibrium of 0.9 within five minutes. This is supportive of the assumption that the uptake of anaesthetic agent is constant after the gas concentration in the entire system reaches equilibrium.
Low flows also prevent wastage of gases, which is beneficial when using agents, like sevoflurane, which have a low uptake and require to be administered in high amounts to achieve a high partial pressure in the system and provide adequate depth of anaesthesia [1].
There is no generally accepted vaporiser dial setting in low flow anaesthesia and the only existing theoretical model has not been experimentally validated [21]. In our study, we attempted to standardise the vaporiser dial setting of sevoflurane while using low flows with air and oxygen mixture in order to avoid complex calculations. The dial settings were altered in increments or decrements of 0.5 whenever the heart rate or systolic blood pressure increased or decreased by 20%, as well as to maintain a MAC of 1 to 1.2.
Nitrous oxide is an integral part of general anaesthesia as it provides intra-operative analgesia and anaesthesia. But there is a theoretical risk of delivering a hypoxic mixture when using N2O at low flow rates. Although many safety systems and monitors like oxygen ratio controller and link 25 have been developed and incorporated in newer anaesthesia machines and workstations to prevent the delivery of such a hypoxic mixture, these machines and monitors are not entirely fool proof. Of late, there is a growing concern that the use of N2O might not be safe for the patient, the operating room personnel and the environment, in general. The usage of nitrous oxide has also been associated with expansion of gas filled cavities and postoperative nausea and vomiting and there are several studies linking the usage of nitrous oxide to peri-operative myocardial infarction [22,23].
Owing to these concerns nitrous oxide has been omitted during the administration of anaesthesia in many centres. However, owing to its analgesic and anaesthetic properties, its omission is expected to result in increased analgesic requirements intra-operatively [24]. Nitrous oxide also potentiates rapid induction and elimination of volatile anaesthetic agents, leading to lesser use of volatile anaesthetic agents, thereby reducing the cost of anaesthesia. Our data demonstrated a significant increase in fentanyl requirements in the study group compared to the control group.
Our cost calculations demonstrated a 62% cost increase when nitrous oxide was omitted. This increase in cost is attributed to the increased requirements of sevoflurane when using low flows without nitrous oxide with respect to the increased dial setting required to maintain a MAC of 1-1.2. But this factor can be offset by the fact that an anaesthetic practice that involves nitrous oxide will have to be accompanied by inbuilt safety features and mechanisms to prevent the administration of a hypoxic mixture. The set-up of an effective safety system will result in significant increase in capital expenditure.
Limitation
Our study has several limitations. First, this study was designed as a preliminary study to establish the safety of low flow anaesthesia in the absence of nitrous oxide. The sample size of this study has not been assessed to demonstrate superiority or inferiority of one method over the other. Second, the sevoflurane dial setting arrived at in this study is yet to be validated. A prospective randomised controlled trial with adequate sample size may overcome these limitations.
Conclusion
Our study demonstrated that the use of medical air and oxygen in flows of 300 mL each following initial high flow rates of 3 L each for 10 minutes, for a period of two hours is a safe technique of low flow anaesthesia. Although, this technique resulted in greater analgesic requirement and overall cost per anaesthetic, it obviates the need for expensive monitoring systems required with the use of nitrous oxide mixture in low flow anaesthesia.
* Student’s t-test
*1ml of sevoflurane gives 184 ml of saturated vapour, according to the formula, Fluid (VA) mL = (specific wt) × Avagadro’s constant × (273 + temp) ÷ (mol.wt × 273),
**The formula for the calculation of amount of volatile agent utilised is given by Fluid vol.agent= (mean FGF × mean agent × duration) ÷ (sat.gas vol × 100)
***The cost of amsorb is not included in the analysis as there is no difference in the fresh gas flows.