Plasma Exchange as a Therapeutic Modality in a Rare Case of Cryptogenic New Onset Refractory Status Epilepticus (NORSE)
Manisha Shrivastava1, Smita Chouhan2, Seema Navaid3
1 Professor, Departmemt of Transfusion Medicine, Bhopal Memorial Hospital and Research Center, Bhopal, Madhya Pradesh, India.
2 Senior Consultant, Department of Transfusion Medicine, Bhopal Memorial Hospital and Research Center, Bhopal, Madhya Pradesh, India.
3 Medical Officer, Department of Transfusion Medicine, Bhopal Memorial Hospital and Research Center, Bhopal, Madhya Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Manisha Shrivastava, A-9 Bhopal Memorial Hospital and Research Center Campus, Bhopal-462038, Madhya Pradesh, India.
E-mail: manishasdr@gmail.com
Refractory Status Epilepticus (RSE) not responding to any therapy and not associated with any aetiology has been termed as New Onset Refractory Status Epilepticus (NORSE). Guidelines for optimal management of cryptogenic NORSE are not clearly defined so far in the literature. Other than common medication, use of high-dose steroids, IV immune globulins and plasma exchanges in NORSE of unknown aetiology have been scarcely described. Immunomodulatory therapy like plasmapheresis is based on the fact that a pathological substance exists in the plasma that contributes to the disease process and its symptoms, which gets removed. We report a case of young female patient diagnosed as NORSE who responded to treatment with plasma exchange after becoming refractory to antiepileptic therapy and treatment with anaesthetic agents for recurrent seizers.
EEG, Epilepsy, Glassgow coma scale, Immunotherapy, Plasmapheresis
Case Report
A 24-year-old female patient was brought to the Emergency Department of our institution in intubated condition and was first assessed by the emergency staff. She was referred to the neurology department and was examined as per the Glasgow Coma Scale (GCS), eye opening to painful stimulus, with no verbal response and no motor response (GCS-E3VT M1). She was admitted in the neurology ward and was shifted to intensive care unit of our hospital and required ventilator support. She had history of sudden onset of convulsions 15 days back when she developed involuntary movements of upper and lower limbs associated with up rolling of eyes. She was admitted in another hospital where she was treated with anti-epileptic drugs- phenytoin, carbamazepine and sodium valproate along with mannitol infusion and steroids, antiviral, antibacterial, acyclovir, vancomycin being tentatively diagnosed as viral encephalitis with status epilepticus. During the course of treatment she initially showed improvement with infrequent episodes of convulsions. Cerebrospinal Fluid Study (CSF) was done and no abnormality was detected during hospitalization. Before the first episode, she had no risk factors, family history or prior history of seizures or fever. She again developed uncontrolled seizures with up rolling of eyes and non-responsiveness to anti-epileptic medicines. She could not maintain oxygen saturation (SpO2) and was intubated and referred to our institution for treatment. Further investigations were done and her EEG, MRI and NCCT were normal. During her course of stay she was treated with dexamethasone-4 mg TDS, carbamazipine- 300 mg QID, phenytoin- 500 mg loading dose and maintenance dose of 100 mg TDS, sodium valproate -250 mg BD, mannitol-100 ml TDS, acyclovir.
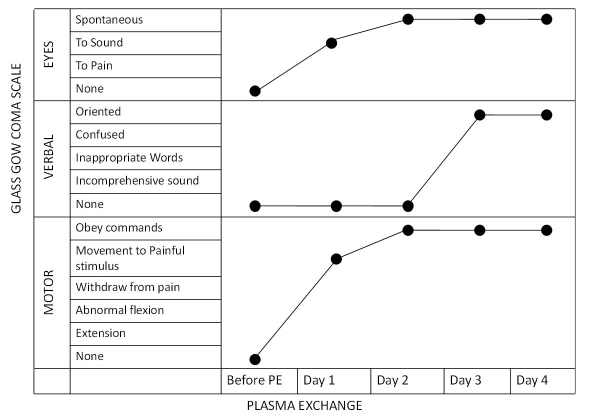
However, there was no improvement. On 5th day of admission she was diagnosed as cryptogenic NORSE by department of neurology due to unknown aetiology and inconclusive investigations and the patient was non responsive to a combination of steroids, antibiotics and antivirals and anti-epileptics as well stroke, brain mass, infective aetiology like causes were ruled out. As a treatment option therapeutic plasma exchange was considered and the patient was subjected to plasma exchange on 6th day of hospitalization. After the first cycle of plasma exchange she showed improvement with eye opening in response to sounds and limb movements in response to painful stimulus. Her GCS score improved to E3M4VT (7/15) with decrease in frequency of seizures. Patient had spontaneous eye opening and no seizure episodes after the completion of second cycle of plasma exchange with a GCS- E4M4VT (8/15). Following third cycle of plasma exchange, patient was electively extubated and maintained 97% oxygen saturation without any ventilator support. She was conscious, oriented with spontaneous eye opening and obeyed the verbal commands with a GCS of 15/15 E4V6M6. Subsequently carbamazepine, acyclovir, mannitol and dexamethasone intravenous infusions were stopped and patient was continued on sodium valproate and phenytoin infusions. After the fourth cycle of plasma exchange rest of the intravenous anti-epileptics were stopped except the anti-bacterials, ceftriaxone and vancomycin which were continued for three more days. Patient showed no further convulsions and was shifted in day care unit from intensive care unit on 6th day of initiation of plasma exchange. Overall patient had undergone four cycles of plasma exchange with 1.5 times volume exchanged each time, replaced with normal saline and fresh frozen plasma and the course of illness was monitored with GCS [Table/Fig-1]. She did not have any episode of seizure since then and was eventually discharged three days after the last cycle of plasma exchange on two anti-convulsants -phenytoin and valproate along with levatiracetam, citalopram and folic acid supplements. Patient is on regular follow up since last three months with no neurological deficit till date.
Clinical improvement of patient in response to plasma exchange therapy monitored by Glassgow coma scale.
Discussion
RSE has been defined as Status Epilepticus (SE) that fails to respond to standard first and second line of treatment with antiepileptic drugs [1]. Patients with no previous history of epilepsy or identifiable causes have been termed as NORSE [2]. The cases have been associated with significant morbidity and mortality and both duration and number of failed medications determine the definition [3]. Various treatment options include specific medications, ketogenic diet, surgical resection, hemispherectomy, vagal nerve stimulators, adrenocorticotrophic hormone, steroids, hypothermia, brain stimulation and immunomodulatory therapies [4]. Few case series describe the use of high-dose steroids, IV immune globulins and plasma exchanges in NORSE of unknown aetiology [5-7]. Though management of cryptogenic NORSE by therapeutic plasmapheresis has not been included in American Society for Apheresis (ASFA) guidelines, this clinical case highlights the therapeutic challenges associated with RSE and use of plasmapheresis to reduce mortality and morbidity in NORSE. Judy Li et al., used plasma exchange therapy in three patients of NORSE with cryptogenic aetiology; two of them showed no episodes of seizures after the fourth day of plasma exchange along with simultaneous use of anti-epileptics [6]. In a case series reported by Khawaja AM et al., NORSE was diagnosed in 11 patients, among which 8 patients were treated with intravenous steroids, immunoglobulins and plasmapheresis alone or in combination with chemotherapy [7]. Our study also showed that GCS scale could also be used in a clinical setting to follow up the improvement in these patients [8]. The above studies indicate more than 80% success rate with use of plasmapheresis in RSE and no appreciable side effects were noted in patients. In our case patient was refractory to the standard anti-epileptic treatment and after the fourth cycle of plasmapheresis our patient showed complete cessation of seizures similar to studies done by Gall CR et al., and Li J et al., [5,6]. A systematic review identified 22 original articles in relation to RSE treatment and use of plasmapheresis and concluded that adult autoimmune RSE response to PE therapy was uncertain and Oxford level 4, GRADE D evidence exists . A response rate of 51.9% in 27 adult patients was found [9]. Similar conclusion was drawn in a systematic review of 37 paediatric patients [10]. The antineuronal antibodies were not tested in our case and further testing of antibodies might have been revealing however, it could be hypothesised that plasma exchange removed the pathological substances contributing to NORSE. Recent guidelines also suggest use of non-pharmacological options as a part of treatment algorithm. Guidelines for optimal management of cryptogenic NORSE are not clearly defined so far in the literature. Some of the studies suggest that conventional treatment is associated with worse outcome in these cases. Most of these cases have been reported with protracted clinical course, increasing the incidence of morbidity in ICU or are sometimes followed by severe neurological deficit [1-3]. In resource constraint settings and as limited evidence exists, physicians too hesitantly treat patients of NORSE with unknown aetiology with alternate therapies. Our centre regularly does plasma exchange therapy for established evidence based categories after referring to treatment options available through literature search, the decision of starting plasma exchange was taken leading to recovery of the patient.
Conclusion
Though plasmapheresis is not yet a definitive indication for treating cryptogenic NORSE yet it could be of potential benefit to refractory epileptic patients and could reduce morbidity and prevent mortality associated with NORSE. More studies are needed to throw light on the aetiology, course of disease and the treatment options in cryptogenic NORSE.
[1]. Shorvon S, Ferlisi M, The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocolBrain 2011 134:2802-18. [Google Scholar]
[2]. Wilder-Smith EPV, Lim ECH, Teoh HL, Sharma VK, Tan JJH, Chan BPL, The NORSE syndrome: defining a disease entityAnnals of the Academy of Medicine (Singapore) 2005 34:417-20. [Google Scholar]
[3]. Van Lierde I, Van Paesschen W, Dupont P, Maes A, Sciot R, De novo cryptogenic refractory multifocal Febrile status epilepticus in the young adult: a review of six casesActa Neurologica Belgica 2003 103:88-94. [Google Scholar]
[4]. Abend NS, Dlugos DJ, Treatment of refractory status epilepticus: literature review and a proposed protocolPediatric Neurology 2008 38(6):377-90. [Google Scholar]
[5]. Gall CR, Jumma O, Mohanraj R, Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapySeizure 2013 22:217-20. [Google Scholar]
[6]. Li J, Saldivar C, Maganti RK, Plasma exchange in cryptogenic new onset refractory status epilepticusSeizure 2013 22:70-73. [Google Scholar]
[7]. Khawaja AM, Dewolfe JM, Miller DM, Szaflarski JP, New onset refractory status epilepticus (NORSE): the potential role for immunotherapyEpilepsy Behav 2015 47:17-23. [Google Scholar]
[8]. Teasdale G, Jennett B, Assessment of coma and impaired consciousness. A practical scaleLancet 1974 2(7872):81-84. [Google Scholar]
[9]. Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM, Plasmapheresis for refractory status epilepticus, part I: A scoping systematic review of the adult literatureSeizure 2016 43:14-22. [Google Scholar]
[10]. Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM, Plasmapheresis for refractory status epilepticus, part II: A scoping systematic review of the pediatric literatureSeizure 2016 43:61-68. [Google Scholar]