Cerebral malformations are of fundamental importance in the clinical diagnosis of Aicardi Syndrome (AS). Some of these anomalies like callosal agenesis, cysts formation, posterior fossa anomalies and gross interhemispheric asymmetry are easily observed in the prenatal period with the use of foetal Magnetic Resonance Images (MRI). We present two cases of female newborns with cerebral MRI performed in the prenatal period and further diagnosed with AS. With the increase use of foetal MRI, AS will be easier suspected in the prenatal period in a female fetus with typical brain anomalies.
Case 1
A 23-year-old healthy woman, (G2, P1), was referred to our hospital for foetal MRI to confirm and evaluate the hydrocephaly diagnosed by ultrasonography at 32 weeks of pregnancy. There was no consanguinity and she has a healthy daughter from her previous marriage.
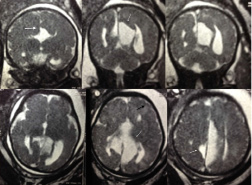
The MRI performed at 35 gestation weeks showed a dysmorphic brain with hydrocephaly, agenesis of corpus callosum, cystic formations and dysmorphic cortex and cerebellum [Table/Fig-1].
Foetal MRI performed at 35 gestation weeks (case 1). Coronal (first row) and axial (second row) T2 weighted images show dysmorphic brain with corpus callosum agenesis (asterisk), hemispheric asymetry and cysts (arrow).
A female of 39 weeks gestation time was born by caesarean due to the confirmation of hydrocephaly. The newborn weight was 2470gram and the Apgar score 8 and 9 at 1 and 5 minutes respectively. A chest x-ray scan revealed lumbar hemivertebra; a fundoscopic examination revealed bilateral chorioretinal lacunae compatible with the diagnosis of AS.
A ventricular peritoneal shunt was inserted at 25 days of life because of progressive hydrocephaly. At six weeks of life, the patient developed focal seizures and few days later she developed epileptic spasms. The electroencephalography revealed high voltage spikes and polyspikes and voltage attenuations bilaterally and independently in both hemispheres compatible with alternant hipsarrhythmia.
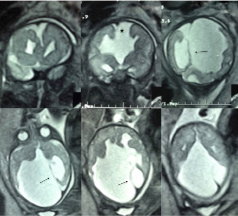
A MRI was performed at seven months of life and showed corpus callosum agenesis, a large posterior fossa cyst, dysmorphic cortex compatible with polymicrogyria and gross interhemispheric asymmetry [Table/Fig-2].
MRI performed at seven months of life (case 1). Coronal (first row) and axial (second row) T2 weighted images show posterior fossa and interhemispheric cysts (thin arrows), corpus callosum agenesis (asterisk) and polymicrogyric cortex (thick arrow).
She developed refractory epilepsy and profound developmental delay with poor visual contact and without babbling or cephalic control at 18 months of age and eventually died because of a respiratory infection when she was two-year-old.
Case 2
A 32-year-old woman, G 2, P1, with a previous healthy daughter was scanned with foetal MRI at 36 weeks gestation because of a sonographic diagnosis of agenesis of the corpus callosum performed at seven months of gestation. Her personal and family histories were unremarkable and she had a nonconsanguineous marriage.
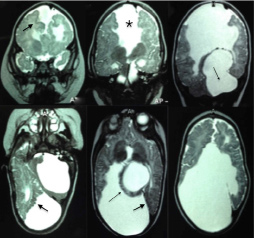
The foetal MRI showed corpus callous agenesis, midline cysts, interhemispheric asymmetry, posterior fossa anomaly and probable dysmorfic cortex [Table/Fig-3]. A female of 38 weeks gestation time was born by an uneventful vaginal delivery. The birth weight was 2750 g and Apgar score was 9 and 10 at 1 minute and 5 minutes respectively.
Foetal MRI performed at 36 gestation weeks (case 2). Coronal (first row) and axial (second row) T2 weighted images showing corpus callosum agenesis (thick arrow), midline cysts (thin arrow), hemispheric asymmetry, posterior fossa anomaly and probable dysmorphic cortex (black arrow).
Because AS was among the possible differential diagnoses based on MRI findings, funduscopy was performed on the second day of life showing chorioretinal lacunae bilateraly. Postnatal MRI could not be performed because the patient did not attend outpatient controls.
Discussion
AS, first described by Jean Aicardi in 1965 [1], is a rare disorder with an estimated incidence between 1 per 93000 and 1 per 105000 live births [2] and a classical triad composed by agenesis of corpus callosum, chorioretinal lacunae and infantile spasms, associated with numerous comorbidities such as profound developmental delay and intractable epilepsy [3].
The causative factor in this disorder, which affects females exclusively, is presumed to an X-linked dominant de novo mutation, but no candidate genes have been identified.
Because of the inability to diagnose with molecular tools, the diagnosis of AS is made using criteria proposed by Jean Aicardi [4]. Using these diagnostic criteria, certain brain anomalies are of fundamental importance: total or partial agenesis of corpus callosum, cortical malformations, periventricular and subcortical heterotopias, intracranial cysts and gross hemispheric asymmetry and their conjunctive presence is highly suggestive of AS [4,5].
Postnatal images in AS have been extensively reported in the literature [6,7]. However, foetal MRI of AS patients are scarce [8-10]. Hopkins B et al., [11] reviewed 23 post natal brain MRI of patients with AS and polymicrogyria, heterotopias and posterior fossa anomalies were present in 100% of the patients and cysts in 95%. Callosal agenesis or dysgenesis was also present in 100% of the patients. (But it was not reviewed in the paper because the authors considered it an inclusion criteria for the diagnosis of AS).
Within these anomalies included in the diagnostic criteria, corpus callosum agenesis, intracranial cysts and gross hemispheric asymmetry are easily observed in foetal MRI. Posterior fossa anomalies, frequently observed in AS, are also easily recognized by MRI performed during the prenatal period.
In case 1 patient had a diagnosis of AS performed in the first month of life due to the agenesis of corpus callosum and the pathognomonic eye anomalies, further supported by postnatal MRI and the epileptic disorder.
Nevertheless, we think that the diagnosis could have been suspected in the prenatal period with the MRI images that showed a female fetus with callosal agenesis, cysts formations, posterior fossa anomalies and interhemispheric asymmetry. These anomalies presenting together are suggestive of AS. Furthermore, Barkovich AJ et al., suggested that the absence of corpus callosum in association with cysts is highly suggestive of AS [7].
In case 2, AS was prenatally suspected due to foetal MRI with corpus callosus agenesis, interhemispheric cysts and posterior fossa anomaly and confirmed in the second day of life with funduscopy.
There are previous reports of patients with prenatal ultrasound, one of them with two cases with macrocephaly with cysts [12] and other with suspected intrauterine hydrocephalus [11], both later diagnosed with AS in the postnatal period. Additionally there are three previous reports with foetal MRI. One patient with prenatal MRI performed at 30 weeks of gestation with cyst of quadrigeminal cistern [8] and postnatal diagnosis of AS. Another case with serial foetal MRI performed at 22 and 33 weeks of gestation with mild ventriculomegaly, complete agenesis of the corpus callosum and right frontal polymicrogyria [9]. And another report of two cases with distortion of inter hemispheric fissure [10], one patient with choroid plexus cysts and thin and short corpus callosum and the other one with bilateral ventriculomegaly associated with irregularity of right ventricular wall, ventricular parallelism and midline cystic structure. Each of these shared similarities with our cases.
Because the lack of prenatal diagnostic tool, the diagnosis of AS is made in the postnatal period with the use of ophthalmologic examination and images of the central nervous system and supported with the evaluation of the epileptic disorder and other suggestive anomalies. Although the ophthalmological examination is currently essential for the diagnosis, making impossible prenatal confirmation, with the broad use of foetal MRI, AS could be suspected in utero allowing early diagnosis in the first days of life.
Conclusion
AS is a rare disorder diagnosed postnatally by typical cerebral and ophthalmologic anomalies. With the increase use of foetal MRI, AS will be easier suspected in the prenatal period in a female fetus with typical brain anomalies. The inclusion of AS as a possible diagnosis in selected fetus with typical cerebral anomalies allow early confirmation of the disease in the neonatal period.
[1]. Aicardi J, Lefebvre J, Lerique-Koechlin A, A new syndrome: spasms in flexion, callosal agenesis, ocular abnormalitiesElectroencephalogr Clin Neurophysiol 1965 19:609-10. [Google Scholar]
[2]. Kroner BL, Preiss LR, Ardini MA, Gaillard WD, New incidence, prevalence, and survival of Aicardi syndrome from 408 casesJ Child Neurol 2008 23:531-35. [Google Scholar]
[3]. Rosser TL, Acosta MT, Packer RJ, Aicardi syndrome: spectrum of disease and long-term prognosis in 77 femalesPediat Neurology 2002 27:343-46. [Google Scholar]
[4]. Aicardi J, Aicardi syndromeBrain & development 2005 27(3):164-71. [Google Scholar]
[5]. Cabrera MT, Winn BJ, Porco T, Strominger Z, Barkovich AJ, Hoytt CS, Laterality of brain and ocular lesions in Aicardi syndromePediat Neurology 2011 45:149-54. [Google Scholar]
[6]. Hall-Craggs MA, Harbord MG, Finn JP, Brett E, Kendall BE, Aicardi syndrome: MR assessment of brain structure and myelinationAm J Neuroradiol 1990 11:532-36. [Google Scholar]
[7]. Barkovich AJ, Simon EM, Walsh CA, Callosal agenesis with cyst. A better understanding and new classificationNeurology 2001 56:220-27. [Google Scholar]
[8]. Columbano L, Luedemann W, Kusaka Y, Oi S, Samii M, Prenatal diagnosed cyst of the quadrigeminal cistern in Aicardi syndromeChild Nerv Syst 2009 25:521-22. [Google Scholar]
[9]. Hergan B, Atar OD, Poretti A, Huisman TA, Serial foetal MRI for the diagnosis of Aicardi syndromeNeuroradiol J 2013 26:380-84. [Google Scholar]
[10]. Vinurel N, Van Nieuwenhuyse A, Cagneaux M, Garel C, Quarello E, Brasseur M, Distortion of the anterior part of the interhemispheric fissure: significance and implications for prenatal diagnosisUltrasound Obstet Gynecol 2014 43(3):346-52. [Google Scholar]
[11]. Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G, Neuroimaging aspects of Aicardi SyndromeAmerican Journal of medical genetics part A 2008 146A:2871-78. [Google Scholar]
[12]. Bromley B, Krishnamoorthy KS, Benacerraf BR, Aicardi syndrome: prenatal sonographic findings. A report of two casesPrenat Diagn 2000 20:344-46. [Google Scholar]