EC is one of the commonest gynaecological malignancy worldwide. The incidence is expected to rise due to a global increase in the prevalence of risk factors for EC i.e. obesity and diabetes [1-2].
Most cases of EC are diagnosed at an early stage with good prognosis. Fortunately, 70% of EC are diagnosed in initial stages due to early presentation of symptoms, the most frequent of which is abnormal vaginal bleeding from the uterus [3]. However, about 30% of ECs occur in asymptomatic women or are diagnosed at an advanced stage [4]. Presentation may be unusual at times. This particular subgroup of patients would certainly benefit from the advent of a reliable serum marker panel to aid early diagnosis.
EC patients with low-grade tumours, endometroid histology and no/only inner-half Myometrial Invasion (MI) are at negligible risk of Lymph Node Metastases (LNM) and may be spared from the morbidity of lymphadenectomy [4]. However, identification of these patients before surgery remains challenging. A prognostic marker that may preoperatively predict the stage of disease, lymph nodal or myometrial involvement would help to plan treatment in a more individualized fashion.
The role of tumour markers in EC is still debatable. Although, Carcinoma Antigen-125(CA-125) is commonly used biomarker, it has poor sensitivity and specificity [5]. Only 10% to 20% of patients with early stage EC and approximately 25% of patients with asymptomatic recurrent disease have an elevated CA-125 level [6-7]. Elevated CA-125 levels have been demonstrated to correlate with advanced disease [8].
HE4 is a novel tumour marker. Preliminary data has demonstrated overexpression of HE4, also known as WFDC2 in EC. Several studies have been published in recent years about use of serum HE4 as diagnostic and prognostic EC biomarker [9-11].
Unfortunately, to date, no good marker for EC is available that may be routinely used in clinical practice for detection, prognosis and monitoring of EC. Thus, the challenge to find a preoperative tool for EC diagnosis and staging is still open. The present study was planned to assess the utility of serum HE4 as a biomarker for diagnosis of EC and to investigate its association with clinicopathological variables.
Materials and Methods
We conducted a pilot study of 60 patients treated surgically for primary EC between October 15, 2014 and January 15, 2016 at Department of Obstetrics and Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India, in collaboration with Institute of Cytology and Preventive Oncology, Noida, Uttar Predesh, India. Women aged 50–79 years, diagnosed with EC during this period and scheduled for surgical staging were prospectively recruited after obtaining informed consent. The exclusion criteria included: (1) patients unfit for surgery, (2) presence of a secondary malignancy, (3) Concomitant benign and/or malignant adnexal pathologies, (4) history of abdominal Koch’s, (5) women who have received treatment for malignancy earlier. The Institutional Review Board approved the study. Sixty age matched healthy postmenopausal women were included in the study as control in which serum level of tumour markers was measured.
About 10 ml of peripheral blood venous sample was obtained preoperatively from each patient and control subjects and examined for CA-125 and HE4. All sera were acquired following a standard collection protocol. Samples were collected in a vacutainer, clotted 60–90 minutes and centrifuged for 10 minutes at 1,300×g. Serum fractions were aliquoted and stored at −80 °C until analysis. Specimens were analyzed at Institute of Cytology and Preventive oncology, Noida by means of chemiluminescent micro particle immunoassays specific for HE4 (ARCHITECT HE4 assay; Abbott Diagnostics, Lane Cove, NSW, Australia) or CA-125 (ARCHITECT CA-125II assay; Abbott Diagnostics, Lane Cove, NSW, Australia). The interassay imprecision values for CA-125 were 2.69% (37.9 kU/L), 2.6% (292.9 kU/L), and 1.26% (612 kU/L). Interassay precision values for HE4 were 3.6% (49.3 pmol/L), 4.6% (171.8 pmol/L), and 4.5% (662.8 pmol/L).
For this study, we considered a standard HE4 cutoff value of 70 pmol/L and CA-125 cutoff of 35U/ml, according to the manufacturer’s indications, and also as suggested by Moore RG et al., [7]. The area under the ROC curve (AUC) was calculated to determine our own cut off values which incidentally were close to the standard values.
Patients were staged according to International Federation of Gynaecology and Obstetrics (FIGO) and Gynaecologic Oncology Group staging procedure surgical staging system. Postoperatively, the histopathological examination was performed and reported according to WHO histopathological classification for EC [12].
Mean value of tumour markers were calculated in EC cases and control group. Data was further analyzed according to stage, histopathology, MI and lymph node involvement. The ability of the diagnostic models to detect malignancy was tested prospectively and compared with the final histopathological diagnosis.
Statistical Analysis
Statistical analysis was performed by the SPSS program for Windows, version 20.0. Continuous variables were presented as mean ± SD, and categorical variables were presented as absolute numbers and percentage. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t-test, whereas Mann-Whitney U test was used for those variables that were not normally distributed. Categorical variables were analyzed using either the Chi-square test or Fisher’s exact test. The AUC with 95% confidence interval, sensitivity, and specificity were calculated to analyze the diagnostic accuracy of CA-125 and HE4. For all statistical tests, a p-value of less than 0.05 was considered significant.
Results
Clinical and pathological characteristics of patient cohort have been presented in [Table/Fig-1]. Mean parity in the patients with EC was significantly lower than healthy controls -(2.26± 0.99 vs. 3.53± 1.10, p-value<0.001); whereas mean BMI in EC cases was significantly higher than the control group (27.4 ± 3.11kg/m2 vs. 22.3±2.71kg/m2, p-value<0.05). Out of the total 60 patients with EC, 20 (33%) were associated with diabetes mellitus, 12 (20%) with hypertension and 20 (33%) with obesity in comparison to controls in which 6 (10%), 8 (13%), 10 (17%) were associated with diabetes mellitus, hypertension and obesity respectively. This difference was statistically significant for diabetes mellitus with a p-value of 0.05. Comparative analysis of the serum HE4 levels and CA-125 for each group is given in [Table/Fig-2].
Clinical and pathological characteristics of patient cohort.
| Clinical characteristics |
|---|
| Parameters | Endometrialcancer (n=60) | Control(n=60) | p-value |
|---|
| Age (Mean+SD)Parity (Mean+SD)BMI (kg/m2) | 62.77+6.612.26 ± 0.9927.4 ± 3.11 | 60.77+7.113.53 ± 1.1022.3 ±12.71 | 0.264<0.001<0.05 |
| Pathological characteristics |
| Histology | N (%) |
| Endometroid adenocarcinomaNon-endometroid carcinomaMucinous carcinomaPapillary serous carcinomaClear cell carcinomaOthers | 36 (60%)24 (40%)06 (10%)14 (23%)04 (07%)00 (00%) |
| FIGO Stage | N (%) |
| Stage IStage IAStage IBStage IIStage IIIStage IV | 42 (70%)26 (43%)16 (27%)12 (20%)06 (10%)00 (00%) |
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4
Comparative analysis of Serum HE4† and CA-125* levels for each group.
| Pathological Characteristics | Serum CA-125 | Serum HE4 |
|---|
| Mean ± SD (U/ml) | Number of Cases aboveCut-off | Mean ± SD (pmol/l) | Number of Cases aboveCut-off |
|---|
| Endometrial Cancer (n=60) | 59.69 ± 77.50 | 22/60 (36.66%) | 127.07±61.36 | 50/60 (83.33%) |
| Healthy Control (n=60) | 18.65±7.63 | | 38.71±9.05 | |
| p-value | 0.005 | | 0.001 | |
| Histology |
| Endometroid Adenocarcinoma (n=36, 60%) | 47.3± 35.38 | 11/36 (30.55%) | 118.6±52.2 | 29/36 (80.55%) |
| Non-Endometroid Adenocarcinoma (n=24, 40%) | 73.19±83.23 | 11/24 (45.83%) | 141.01±72.9 | 21/24 (87.5%) |
| p-value | 0.328 | | 0.205 | |
| Myometrial Invasion (Stage I) |
| Stage IA (<50%) (n=26, %) | 33.59±47.57 | 02/26 (7.69%) | 109.92±54.07 | 24/26 (88.46%) |
| Stage 1B (>50%) (n=16, %) | 93.83±93.75 | 07/16 (43.75%) | 150.72±63.61 | 11/16 (68.75%) |
| p-value | 0.023 | | 0.007 | |
| Lymphnode Involvement |
| Pelvic lymphnode (n=60, 100%) | | | | |
| Present (n=6, 10%) | 209.33±92.14 | 06/06 (100%) | 248.30±45.76 | 06/06 (100%) |
| Absent (n=54, 90%) | 43.07±54.27 | 16/54 (29.62%) | 114.19±46.49 | 44/54 (81.48%) |
| p-value | 0.01 | | 0.006 | |
| Para-aortic Lymphnode | | | | |
| Present (n=0, 0%) | - | | - | |
| Absent (n=60, 100%) | 59.69±77.50 | | 127.07±61.36 | |
| Stage | | | | |
| Stage I (n=42, 70%) | 30.03±33.79 | 09/42 (21.42%) | 112.53±46.88 | 35/42 (83.33%) |
| Stage II (n=12, 20%) | 88.70±83.77 | 07/12 (58.33%) | 120.02±46.63 | 09/12 (75%) |
| Stage III (n=6, 10%) | 209.33±92.15 | 06/06 (100%) | 248.30±45.76 | 06/06 (100%) |
| p-value | | | | |
| Stage I vs II | 0.020 | | 0.739 | |
| Stage II vs III | 0.007 | | 0.05 | |
| Stage I vs III | <0.001 | | <0.001 | |
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4
The mean serum HE4 (EC 127.07±61.36 pmol/L vs. 38.71±9.05 pmol/L in healthy controls, p=0.001) and CA-125 levels (EC 59.6±77.5 U/ml vs.18.6±7.63 U/ml in healthy control, p=0.005) of EC patients were significantly higher when compared with healthy controls.
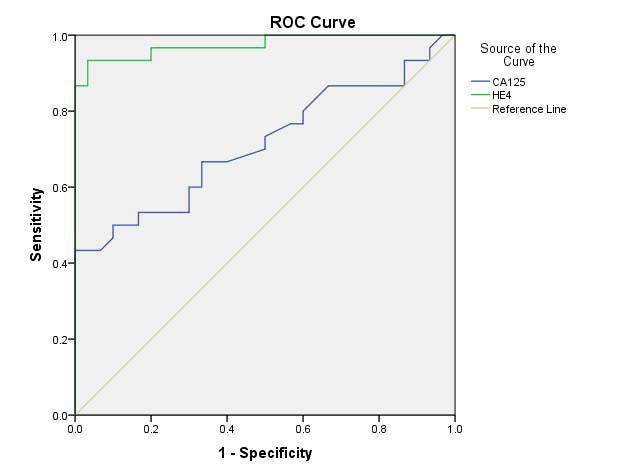
Diagnostic value of serum HE4 and CA-125 for endometrial cancer: ROC curve for HE4 and CA-125 was generated to predict endometrial malignancy. The analysis revealed pretreatment serum HE4 cut off value of 69.7 pmol/l to predict endometrial malignancy (AUC 0.974; SE 0.013; CI −95 to 95 % = 0.951–1.000; sensitivity 86.7%, specificity 100%). Corresponding cut off for serum CA-125 was 34.50 U/mL (AUC 0.714; SE 0.048; CI −95 to 95% = 0.620–0.807; Sensitivity 43.3%, specificity 100%). Serum HE4 levels above the cut off were detected in 50 out of 60 EC patients and CA-125 levels in 22 out of 60 EC cases [Table/Fig-2,3].
Comparative analysis of diagnostic value of CA-125† and HE4*.
| Parameters | AUC | Sensitivity | Specificity | Cut offValue |
|---|
| Serum HE4 | 0.974 (CI, 0.951-1.00), SE 0.013 | 86.7% | 100% | 69.72 pmol/L |
| Serum CA-125 | 0.714 (CI, 0.620-0.807), SE 0.048 | 43.3% | 100% | 34.50 U/L |
| Serum HE4+CA-125 | - | 86.7% | 100% | - |
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4
Comparative analysis of diagnostic value between serum HE4 and CA-125 for endometrial cancer: The ROC analysis revealed that HE4 is superior to CA-125 in differentiating postmenopausal healthy controls [Table/Fig-4]. The sensitivity of HE4 in detecting EC patients was higher when compared to CA-125. The specificity of both HE4 and CA-125 was 100% [Table/Fig-3]. The diagnostic performance was compared between serum HE4, CA-125 and combined HE4+CA-125 values. The sensitivity of combination of HE4 and CA-125 to detect EC was same as that of HE4 [Table/Fig-3].
Receiver Operator Characteristics (ROC) curve of pretreatment a) Serum HE4 and b) CA-125 used as a predictor of diagnosis of endometrial carcinoma.
CA-125, carbohydrate antigen 125; HE4, human epididymis secretory protein 4
Association between serum HE4 and CA125 levels, clinical stages and pathological type of EC: On comparing, HE4 and CA125 levels between EC stages I vs. II/III, the difference was statistically significant (p<0.05). The levels of serum HE4 and CA-125 were higher in the non-endometroid histology; but the difference was not statistically significant [Table/Fig-2].
Analysis of the associations between serum HE4 levels, myometrial invasion and lymphnodal involvement of endometrial cancer: Serum HE4 and CA-125 levels were significantly higher in Stage I EC with >50% MI when compared to <50% MI (p<0.05). Patients with lymphnodal involvement also had significantly higher serum HE4 and CA-125 levels [Table/Fig-2].
Discussion
The present study was conducted to evaluate the clinical utility of HE4, a novel tumour biomarker, for diagnosis of EC and its association with high risk factors in Indian population.
Studies recently demonstrated that serum HE4 and CA-125 levels are significantly elevated in patients with EC [5-11]. Our data confirm these results. The present study indicated that the levels of serum HE4 and CA-125 were significantly higher in a preoperative endometrial tumour group compared with healthy postmenopausal women (p<0.05).
Our results showed that the HE4 has the AUC of 0.974 (0.951-1.00), SE-0.013 for detection of EC. Corresponding AUC for CA-125 was 0.714 (0.620-0.807), SE-0.048. The maximum diagnostic value for the diagnosis of EC occurred when the cut off value of HE4 was 69.7 pmol/l. Unfortunately, no clear cut off value for HE4 serum levels has been defined for detection of EC patients till date. In fact, for detection, only a few studies reported a cut off value, which incidentally differed from study to study. However, based on the meta-analysis by Bie Y and Zhang Z the best HE4 cut-off in EC diagnosis ranged between 50 and 70 pmol/L, resulting in 78.8% sensitivity and 100% specificity for all stages [13].
In our study, the sensitivity of HE4 for detection of EC of all stages was higher than CA-125 (86.7% vs. 43.3%, p<0.05) and specificity was 100% for both CA-125 and HE4, when compared to healthy control. The results of the present study are consistent with that of Angioli R et al. and Moore RG et al., who demonstrated that HE4 as a single marker exhibits higher sensitivity in detecting all stages of EC when compared to CA-125 [5,7].
There is a vast variation in sensitivity and specificity among studies which could be explained by different methods of estimation or different cut off values of tumour biomarkers used. Characteristics of patient population also results in heterogeneity of the diagnostic accuracy of biomarkers. Our study reported higher sensitivity of HE4 in detecting EC when compared to Angioli R et al., (59.4% at a specificity of 92%) and Bignotti et al., (67% at a specificity of 95%) [9,10]. Higher sensitivity of HE4 in detecting EC was probably due to inclusion of all histologies of EC with higher levels in non endometroid type; whereas Bignotti E et al. considered only endometroid EC [10].
Mean value of HE4 and CA-125 increases with stage of EC. There was a statistically significant difference comparing mean value of HE4 and CA-125 in EC patients in Stage I vs Stage II/III and Stage II vs Stage III patients (p-value<0.05) [Table/Fig-2]. Our results are in accordance with other authors who observed that the mean levels of CA-125 were not elevated in early EC; whereas the levels of HE4 had significantly increased in early stage EC [7,14,15]. Thus, CA-125 was not useful in diagnosis of early EC. Zhang W et al., compared EC patients and a healthy control group and concluded that riseof HE4 occurred earlier than that of CA-125 [16].
We also found that the diagnostic value of HE4 was superior to that of CA-125 in Stage I EC patients, where HE4 detected 83.33% (35/42) cases;while 21.42% (9/42) of cases were detected by CA-125, whereas in Stage II, 75% (09/12) and 58.33% (7/12) cases were detected by HE4 and CA-125 respectively. In Stage III, all (100%) cases were detected by both CA-125 and HE4. There were no stage IV EC cases. Angioli R et al., reported elevated CA-125 levels in 19.8% EC cases of Stage I [5]. Beck EP et al., demonstrated that only 15% of Stage I uterine cancer patients, 33% of Stage II, and 62% of Stage III patients had elevated CA-125 levels [17].
Capriglione S et al., observed that 42% of Stage I A, 77% of IB, 90% of Stage II, 93% of Stage III and 100% of Stage IV patients presented with HE4 levels above the standard cut off of 70 pmol/L [18]. On the contrary, Kalogera E et al., demonstrated in his study that HE4 was not able to differentiate between early stage EC patients and controls (p=0.49); thus raising the question regarding its value as a screening tool for EC or detection of early recurrence [14]. Results of our study demonstrated the potential utility of HE4 as a screening tool as it was able to diagnose 23/26 (88.46%) of Stage I A EC cases in comparison to 2/26 cases by CA-125.
However, the sensitivity of HE4 (71%) in detecting advanced stage patients was higher when compared to the sensitivity of CA-125 (58%) as per Saarelainen SK et al., [19]. Angioli R et al., in his critical review on HE4 performance in EC, concluded that the major advantage of HE4 lies in its specificity and improved detection of early stage EC as in case of ovarian and tubal cancers reported by Jacob F et al., [9,20].
Results of present study and other studies also suggest that serum HE4 and CA-125 may offer preliminary risk stratification prior to surgery [17, 20-22]. A serum marker that could provide pretreatment estimation of early stage and low risk disease would potentially find clinical application in the management strategy of identification of suitable candidates with absent or superficial MI and lymph nodal involvement.
Various investigators demonstrated that pre-surgical HE4 and CA-125 markers were elevated in endometrial carcinoma patients with MI [8,11,18]. The results of the present study were in agreement with previous reports regarding association between HE4 and CA-125 and MI. Patients with Stage IA disease (<50% MI) had a significantly lower mean serum HE4 value than patients with Stage IB disease with >50% MI (109.92±54.07 pmol/L vs. 150.72±63.61 pmol/L; p=0.007). Corresponding mean values of CA-125 were also higher in Stage1B as compared to Stage IA of EC (33.59±47.57 U/mL vs. 93.88±93.75 U/mL; p=0.023).
Moore RG et al., [11] and Brennan DJ et al., [15] observed HE4 to be a better predictor of outer half MI than CA-125. Kalogera et al., also observed that median HE4 levels were significantly elevated in EC patients with MI >50% compared to those with MI≤50% (p<0.001) [14]. The results of the present study demonstrated that the benefit of CA-125 lies in its high specificity for detecting EC patients with <50% MI. Serum CA-125 levels below cut off value indicate an early stage of EC. Twenty four out of 26 Stage I EC cases with MI <50% had serum CA-125 levels below cut off value.
Presurgical CA-125 and HE4 levels were shown to be related to the lymph node metastasis [8, 10]. We also found that significantly higher levels of HE4 and CA-125 in cases with lymph nodal involvement. Infact, there was a statistical significant difference comparing Stage I versus Stage III (p<0.001). Antonsen SL et al., and Bignotti E et al., also found significant differences in the tumour marker levels in patients with LNM versus no LNM (p = 0.013 and p < 0.001, respectively [8,10]. Infact Antonsen SL et al., reported CA-125 to be more precise than HE4 for LNM; whereas Prueksaritanond M et al., found that the performance of serum HE4 in identifying EC patients at low risk and high risk of lymph node metastasis was significantly better than that of CA-125 (AUC 0.88 vs. 0.65, p=0.003) [21]. These findings suggest a potential role of HE4 and CA-125 in EC stage prediction and myometrial involvement and thus identify patients suitable for pelvic and para-aortic lymphadenectomy.
The diagnostic value of tumour biomarkers may vary with the histopathological type. The present study observed that the level of serum HE4 and CA-125 was higher in non-endometroid EC patients; but the difference compared with endometroid types was not statistically significant for serum HE4 and CA-125. Kalogera E et al., and Mutz-Dehbalaie I et al., observed no association between serum HE4 levels and histological variants of EC [14,22]. Contrary to our findings, Omer B et al., reported significantly higher (p<0.001) median HE4 value of 130.7 pmol/L in endometroid compared with HE4 value of 86.8 pmol/L in non-endometroid EC cases [23]. Angioli R et al., also reported higher HE4 values in endometroid EC compared to non endometroid cancers [5].
On combining HE4 and CA-125 (if both were positive, the combination was considered positive), the sensitivity and specificity was as that of serum HE4. Therefore, the present study suggests that the addition of serum CA-125 to HE4 provides no additional diagnostic benefit when compared to HE4 alone. In contrast, Bignotti E et al., and Moore RG et al., reported improved sensitivity for higher stages (Stage II-IV) by combining both the markers [10,11].
However, combination of both biomarkers has a prognostic value. When both HE4 and CA-125 were below cut off value, then all (3/3 EC cases without MI invasion or LN involvement were correctly diagnosed. This shows that normal values of both the tumour marker in histologically confirmed cases on endometrial biopsy specimen indicates good prognosis combination of high serum levels of HE4 > 200 pmol/L and CA-125> 34.5U/ml were observed in all (6/6) Stage III EC cases. When both the markers were significantly high, it represents higher stage with poor prognosis.
In literature, very few studies have evaluated the effect of tumour biomarkers for EC detection and/or monitoring. This is due to the fact that EC is diagnosed early due to vaginal bleeding. As the incidence of EC is increasing, unusual presentation and late diagnosis in advanced stage with poor prognosisis not uncommon. This justifies the need to identify diagnostic and prognostic biomarkers for EC as in the case of ovarian cancer.
Limitation
Limitation of our study is small sample size and inclusion of only postmenopausal women.
Conclusion
In conclusion, our results confirm that HE4 is a sensitive diagnostic serum marker for detection of EC patients, exhibiting a better diagnostic performance compared to CA-125. In particular, good performance of HE4 in diagnosis of early stages EC indicates its usefulness as screening tool, monitor therapy and detect early recurrence. Large prospective clinical studies including both pre and postmenopausal EC cases are certainly necessary to support these findings and to assess the potential of HE4 as a screening modality or new tool for preoperative risk stratification and postoperative surveillance of endometrial cancer patients.
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4
†CA125, carbohydrate antigen 125; *HE4, human epididymis secretory protein 4