Lung cancer remains the leading cause of cancer-related deaths worldwide, despite of improvements in detection methods and treatments. Primary lung cancer has been classified in two distinct classes: Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC). There are three main subtypes of NSCLC, including squamous cell carcinoma, adenocarcinoma and large cell carcinoma [1].
The aims and objectives of the present work were to study the histomorphology of NSCLC, to assess the status of ALK and EGFR and their correlation. This would help to find the subgroup for targeted therapy.
Materials and Methods
This retrospective and prospective study was conducted in the Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, from August 2013 to July 2014.
Sample collection: All cases of NSCLC received in past three years were included in the study. Smoking history was noted from record in all patients. The old cases were taken out from the records of pathology department and the new cases were worked up as they were received. The clinical data of the cases were recorded and H&E stained slide and block were retrieved from the records. All the slides available were reviewed to study the histological features of tumours. About 3 μm-4 μm thick sections were taken from each block for H&E staining, ALK Immunohistochemical (IHC) staining on poly L-lysine coated slide and EGFR staining on poly L-lysine coated slide. Positive control used for ALK was anaplastic large cell lymphoma and for EGFR was human placenta.
Procedure: Sections stained with H&E were used to study the histological features of tumour. Standard protocols were used for H&E staining procedure and for IHC. IHC evaluation was done using Streptavidin Biotin immunoperoxidase method. Staining and evaluation using specific polyclonal antibody to ALK was done.
Immunostaining evaluation: Granular cytoplasmic staining in at least one tumour cell (or more tumour cells) in a sample labelled with an anti-ALK antibody was scored as an ALK-positive sample. Grading was done on scale of 0, (no staining), 1+ (weak intensity staining), 2+ (moderate intensity staining), and 3+ (strong intensity staining). For EGFR membranous and cytoplasmic staining in tumour cells was considered positive.
Statistical Analysis
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical analysis software. The values were represented in Number (%), Mean±SD. Chi-square test was used to analyse the data and p-value was calculated wherever required. A p-value of 0.05 or less was considered as statistically significant.
Results
In our study, total 69 cases of NSCLC were included, endobronchial biopsy was done in 36 (52.17%) and intrathoracic biopsy was done in 33 (47.83%) cases, of which SCC was most common accounting for 71% cases (n= 49), adenocarcinoma was 26.1% (n= 18) [Table/Fig-1] and adenosquamous was 2.9% (n=2). The mean age of patients was 55.26±11.51 years. With respect to smoking habits, 81.16% of our patients were smokers however 18.84% patients were non-smokers.
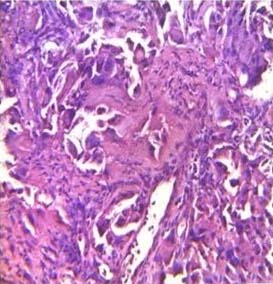
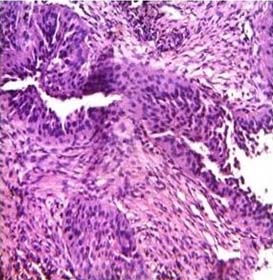
Adenocarcinoma lung (H&E stain, 40X).
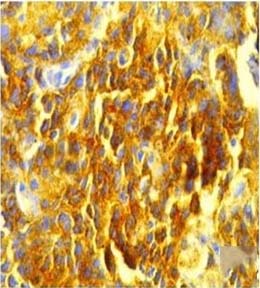
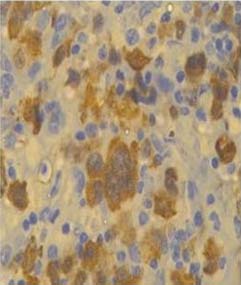
A total of 69 samples were submitted to IHC for detection of ALK and EGFR mutation. Positive control used for ALK was anaplastic large cell lymphoma [Table/Fig-2]. Only one sample was ALK positive (1.45%) [Table/Fig-3]. This ALK positive case was a 60-year-old female, non-smoker patient with a histological diagnosis of lung adenocarcinoma. The case was negative for EGFR mutations also.
Anaplastic large cell lymphoma used as positive control of ALK (IHC stain, 40X).
Tumour cells showing ALK positivity, (IHCstain, 40X).
In our study, EGFR mutation was seen in 63.77% of NSCLC and expression was found to be more in adenocarcinoma (83.33%) [Table/Fig-4], but 59.18% SCC also showed EGFR mutation. Positive control used for EGFR was human placenta [Table/Fig-5]. Although, proportion of patients positive for EGFR was higher in adenocarcinoma group (77.78%) [Table/Fig-6] as compared to adenosquamous carcinoma (50%) and squamous cell carcinoma (59.18%) yet the association was not significant statistically (p=0.343) [Table/Fig-7]. The association between ALK and diagnosis was not significant (p=0.238) [Table/Fig-8].
Adenocarcinoma lung (H&E stain, 40X).
Human placenta used as positive control of EGFR (IHC stain, 40X).
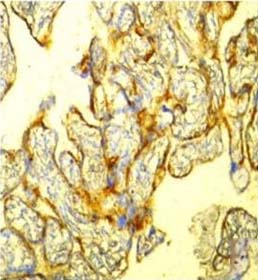
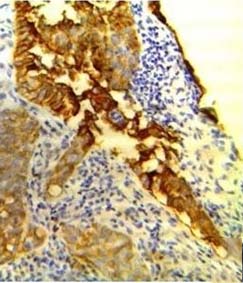
Tumour cells showing EGFR positivity (IHC stain, 40X).
Diagnosis and EGFR findings.
| EGFR Findings | Adenocarcinoma (n=18) | Adeno-squamous carcinoma (n=2) | Squamous cell carcinoma (n=49) |
|---|
| No. | % | No. | % | No. | % |
|---|
| Negative | 4 | 22.22 | 1 | 50.00 | 20 | 40.82 |
| Positive | 14 | 77.78 | 1 | 50.00 | 29 | 59.18 |
p=0.3
Diagnosis and ALK findings.
| ALKFindings | Adenocarcinoma (n=18) | Adeno-squamous carcinoma (n=2) | Squamous cell carcinoma (n=49) |
|---|
| No. | % | No. | % | No. | % |
|---|
| Negative | 17 | 94.44 | 2 | 100.00 | 49 | 100.00 |
| Positive | 1 | 5.56 | 0 | 0.00 | 0 | 0.00 |
p=0.238
Discussion
Lung cancer is common and leading cause of death throughout the world [1]. Our study focussed on NSCLC, which is the leading cause of cancer related deaths in the world. Further subtyping of NSCLC into adenocarcinoma and SCC, large cell carcinoma is important for patient manegment. NSCLC, accounts for approximately 85% of all lung cancers, presenting an epithelial origin [2] whereas the remaining 15% are classified has SCLC. Among NSCLC adenocarcinoma is the most common type of lung cancer in both men and women [3]. In addition to the general category of large cell carcinoma, several uncommon variants are recognized in the WHO/ IASLC classification [3-5]: Large-Cell Neuroendocrine Carcinoma (LCNEC), basaloid carcinoma, lymphoepithelioma-like carcinoma and clear cell carcinoma [3-5].
As compared to Western population, median age of our patients (55.26±11.51 years) was few years younger [6,7] i.e., cancer occurs at early age in Indian population. Most of the previous studies in Indian subjects have reported the similar median age as in ours [8,9]. Median age was in concordance with the study done by Malik PS et al., [10]. In their study, median age was 55 years with a male: female ratio of 4.6:1. In the present study, out of total 69 cases of NSCLC, 52 cases (75.36%) were males and 17 cases (24.64%) were of female patients; male to female ratio being 1:0.33. The increased number of male patients may be because of increased prevalence of smoking in case of male as compared to females. Cigarette smoking is a primary risk factor for the development of lung cancer and is estimated to account for approximately 90% of all cases, which was found in our study also in which 81.16% patients were smokers. Noronha V et al., did a study in 489 patients, in which 77.7% were males and 23.3% were females with a male: female ratio of 3.5:1 [11]. A higher percentage of females (88.1%) were non-smokers, compared to 41.8% of males who were non-smokers. Similar male: female ratio was found in our study. In a study done by Gupta D et al., 89% men and 33% women were smokers compared with 60 % of men and 20 % of women in controls group [12].
In our study, total 69 cases of NSCLC were included, of which SCC was the most common accounting for 71% cases (n=49), adenocarcinoma for 26.1% (n=18) and adenosquamous accounts for 2.9% (n=2) cases. According to several Indian authors, SCC is still the most common subtype [8,9].
ALK-ALK is a trans-membrane receptor with tyrosine kinase activation which belonging to the insulin growth factor receptor superfamily, located on chromosome 2 (2p23). Ligand-independent dimerization of ALK is mediated by multiple fusion partners of ALK resulting in tyrosine kinase activation and then KRAS and PI3K signal pathways activation which leads to anti-apoptotic and cell proliferation signals. In NSCLC carcinogenesis, activation of ALK fused with varies gene partners, but most frequently Echinoderm Microtubule-Associated Protein 4 (EML4) was seen. EML4 gene is located on chromosome 2 (2p21) and is reversely oriented with ALK. EML4-ALK fusion can occur after chromosome cleavage at a variable site and chromosome inversion, which can form various fusion isoforms [13]. EML4-ALK fusions are mutually independent with EGFR or KRAS mutations, means ALK positive cases do not show EGFR or KRAS mutations and almost exclusively seen in adenocarcinoma. The presence of EML4-ALK fusion is more likely in patients with certain characteristics, like never smoker or younger age like in our study, ALK positive case was non-smoker [14]. Most studies have discovered this mutation at a rate of 2%-5% in patients of NSCLC [15-17].
To KF et al., evaluated the usefulness of IHC to detect ALK expression as a trustworthy detection method of ALK rearrangement in lung adenocarcinoma [18]. They examined the role of IHC, to find the ALK fusion. Out of of 373 cases, 22 cases of lung adenocarcinoma (5.9%) were positive for ALK mutation. Tumour cells which were ALK positive in IHC with strong and diffused granular staining in the cytoplasm were further confirmed to having ALK rearrangement, either by FISH, or RT-PCR. Not a single case of ALK IHC-negative was FISH-positive. So IHC can be used as early screening diagnostic test for effective detection of ALK mutation in NSCLC.
In our study, out of total 69 samples, only one sample was positive (1.45%) for ALK and showed a stained region, which, although not as intense as the positive ALCL control, represents abnormal over expression of ALK in this sample type.
Several studies have found that ALK positivity ranges between 1%-8% in NSCLC. A study conducted by Selinger CI et al., found 1% ALK positivity (7/594 cases) which showed concordance with our study [19]. Li Y et al., and Inamura K et al., also showed 3.37% and 3.7% ALK positive cases in their study, respectively [20,21].
EGFR: EGFR gene mutations are commonly found among molecular abnormalities analysed in NSCLCs, particularly in lung adenocarcinomas [22]. The epidermal growth factor receptor (EGFR, HER-1/ErbB1) is a receptor tyrosine kinase of the ErbB family. Its main function is to mediate and control cell signaling by extracellular growth factors, that regulate vital cellular functions like proliferation, angiogenesis and apoptosis [23].
In our study, EGFR mutations were found in 63.77% of all cases of NSCLC. In pioneer study [24], 1482 patients were tested from seven Asian regions, and EGFR mutations were found at a rate of 51.4% overall. While in several published previous studies, this percentage was low especially in western countries [25,26]. Incidence of EGFR mutation was found to be more in adenocarcinoma (77.78%) [Table/Fig-2] in the present study, while 59.18% SCC also showed EGFR mutation. According to studies, EGFR mutations in lung cancer are very strongly associated with adenocarcinoma histology [27]. Similar study was done by Dhanasekar T et al., and they found that 89% of the adenocarcinomas expressed EGFR [28]. According to previous literature this mutation was low in NSCLC other than adenocarcinoma, but in our study, this mutation was also seen in 59.18% of SCC [Table/Fig-2]. Our finding was supported by the study of Arcot R et al., on 38 NSCLC patients. Expression of EGFR in SCC was 80% [29], while in primary and secondary adenocarcinoma EGFR expression it was reported to be 69.6% and 40.0%, respectively.
In literature, EGFR mutations are significantly more frequent in females [27], and these finding were concordance with our study. Arcot R et al., also found the higher frequency of EGFR mutations among women (54%) than men (39%) [29]. The findings were in accordance to a study by Sahoo R et al., who investigated 220 patients; the EGFR mutation status was 50.9% in women and 49.1% in men, p=0.04 [30]. But higher percentage (63.46%) of EGFR mutation is also seen in male cases in our study. In the Pioneer study also, EGFR mutations was found in highest frequency in Asian females (61.1%) and non smokers (60.7%) [24]. However, the percentage was also high in Asian males (44%) and in Asian heavy smokers (30%), which is higher than previous reported percentage of 8.2% in males and 5.8% in heavy smokers in European patients. Thus, physicians should not exclude these other populations from EGFR mutation testing on the basis of clinical characteristics. In our study, 84.62% of EGFR mutations were seen in non-smokers, but smokers also had 60.71% of EGFR mutation.
Patients with EGFR mutations demonstrate response rates higher than 70%, 14-month progression-free survival and 27-month median overall survival when treated with EGFR- tyrosine kinase inhibitors i.e., gefitinib, erlotinib [31,32]. Since 2009, gefitinib was the only licensed drug used in NSCLC with EGFR mutations in all lines of therapy [32]. Recently, erlotinib has been granted US approval as a first-line drug in NSCLC with EGFR mutations. This molecule was already FDA approved for use in maintenance and second-line treatment of NSCLC [33]. With this background, many biomarkers are under study for developing targeted therapy.
Limitation
Limitation of this study is that the sample size was small and in future, larger sample size is needed to further stabilise the correlation specially in Indian population. Smaller percentage of ALK positivity makes analysis difficult in our study. Also, in our study correlation of FISH was not evaluated with IHC positive cases.
Conclusion
We can say that EGFR and ALK expression are mutually exclusive in their expression in NSCLC patients. ALK expression is more likely to be seen in female patients who are non-smokers or have adenocarcinoma in their histology. A distribution pattern of EGFR expression can however not be localised to a particular group of cases. It may be seen in fair number of male or female cases of either adenocarcinoma or SCC. A routine use of these bio-markers is certainly indicated for targeted therapy in the patients of lung cancer. This study provides information on biomarker usage in the Indian population. Such information may be useful to inform policy makers about the utility of biomarkers in NSCLC to improve disease management in India.
p=0.3
p=0.238