Introduction
Nanoparticles composed of Titanium Oxide (TiO2) are non toxic, durable, stable and have a high refractive index with a lot of scope in biomedical applications. Due to their antibacterial effects, they can be applied to inanimate objects like glass, metal and even biomedical implants.
Aim
This study was conducted to assess the antibacterial effect of Titanium Oxide (TiO2) alone or with Silver (Ag) as an additive on Escherichia coli.
Materials and Methods
Escherichia coli isolates (n=25) sensitive to most of the drugs including first generation cephalosporins, ampicillin and amoxycillin from various samples like pus, urine, sputum and blood were placed onto the glass slides containing TiO2 annealed at 200°C, 400°C, TiO2 with 0.1% Ag as additive, TiO2 with 0.3% Ag, and TiO2 with 0.6% Ag as additive. Samples from this were inoculated at every hour onto sterile petri plates and observed for growth after overnight incubation at 37°C.
Results
The organisms which were inoculated onto TiO2 annealed at 200°C showed a slower reduction rate from >1 × 108 cfu/ml to <1 × 10 cfu/ml only after six hours of incubation in visible light. Complete absence of colony forming units was observed after eight hours of incubation. The samples treated with TiO2 at 400°C showed no growth after six hours of incubation itself. Samples treated with TiO2 with increasing gradations of silver as additives showed proportional reduction in the incubation time for the complete absence of colony forming units.
Conclusion
Our study shows that pure titanium oxide has a high antibacterial effect on pathogenic samples of Escherichia coli from clinical isolates, which is further increased with the addition of increasing concentrations of silver.
Introduction
Surgical site infections are the second most common infections among hospital acquired infections causing high morbidity and mortality [1]. They account for a quarter of all nosocomial infections [2]. The infection can be acquired through healthcare workers or any other iatrogenic sources, including scalpels, forceps or body implants during surgeries or any other invasive procedures.
A high incidence rate of infection is observed in the use of implanted biomedical devices, such as bone fracture fixation pins, catheters and heart valves etc., which result in a tremendous increase in human health and healthcare costs [3]. Moreover, all these devices are highly exposed to infection by multiresistant bacteria, on which systemic antibiotics have very little effect [4,5]. For example, Escherichia coli is an especially difficult organism to treat as it can adapt to changing environmental conditions becoming resistant to variety of antibiotics and hence not eradicated completely even after treatment [6].
Hence, an alternative strategy is of utmost priority in order to control infections. Since, bacterial adhesion to biomaterial surfaces is an essential step in the process of infection, modifications to biomedical surfaces are considered to diminish infections by inhibiting initial bacterial adhesion [4,7].
Of late there is a lot of interest in the field of Nanomedicine wherein different types of nanoparticles are used for their antibacterial effect. Nanoparticles are objects of a minute size, measuring about 1-100 nanometers, but have the capacity to behave as a complete unit with respect to its transport and properties. They are further classified according to their shape and size into ultrafine, fine and coarse [8]. Interfacing the nanoparticles with biological molecules and structures may result in a major development in the treatment of various disorders.
In the recent years, nanoparticles, especially those composed of TiO2, is the object of considerable research due to their potential biomedical applications. They are known to be non toxic and highly durable with a high refractive index, apart from having antibacterial properties. Since TiO2 are very stable, it is very easy to apply them to inanimate objects such as glass, metal and biomedical implants.
Different modes of action of the nanoparticles on bacteria have been described. One of them is said to be by oxidative damage on the cell membrane of the organism [9]. Another mode of action described is an alteration of the Coenzyme A dependent enzymes and their activity [10], while damage to the DNA by means of hydroxyl radicals have also been reported to be one of the causes [11].
It is well known that silver ions show good antibacterial properties without any toxic effects in comparison to other heavy metal ions. The biocidal activity of silver is related to the biologically active silver ion released from silver coatings. The silver ions (Ag+), have the capacity to bind to the proteins and enzymes present on the bacterial cell wall and the cell membrane. This leads to the disruption of the cell membrane which causes permeability imbalance, cellular disintegration, ultimately cell death [12,13]. It is also reported that silver has long played a role in the treatment of burns [14]. It has also been noted that the infection rate was effective not only in the humans but also in the animals when treated with silver-coated medicines [15].
Much of the work is done by the researchers on the self cleaning property of TiO2 with or without doping of metal ions in water and in air. Most of the bacterial samples used in these studies are samples of standard strains such as American Type Culture Collection (ATCC) and National Collection of Industrial Microorganisms (NCIM) strains. Nevertheless here has been little work done on samples collected from the clinical samples of the patients.
Hence, in this present study, we have attempted to use Escherichia coli, which is one of the major pathogens of the humans capable of causing diseases from different clinical samples and analyze the antibacterial effect of pure TiO2 and TiO2 with various concentrations of Ag as an additive.
Materials and Methods
This prospective study was performed at the Department of Microbiology of Malla Reddy Institute of Medical Sciences from September 2013 to July 2016.
Inclusion criteria: Twenty five E. coli isolates sensitive to most of the drugs including first generation cephalosporins, ampicillin and amoxycillin from various samples like pus, urine, sputum and blood were included in the study.
Exclusion criteria: All other pathogenic bacteria which were isolated during the culture of the clinical isolates were excluded from the study.
Preparation of nanoprticles: Calculated quantity of titanium isopropoxide (Aldrich, 99.98% purity) was dissolved in 2-methoxy ethanol (99.98%) solvent along with acetyl-acetone as a complexing and chelating agent. 1.2 g of cetyltrimethylammonium bromide was dissolved in 20 ml of 2-methoxy ethanol solvent in beaker and the contents were added drop wise to the titanium isopropoxide sol under vigorous stirring. The contents were stirred at room temperature for about six hours. The molar ratio of titanium isopropoxide, 2-methoxy ethanol and acetyl-acetone were 2.0:10.0:0.5. To the above contents calculated quantities of HNO3 (1.2 ml) and of deionizing water (1.5 ml) were added drop wise as a catalyst to increase the rate of reaction, and hydrolysis of the sol, respectively. Further the contents were refluxed at 70°C for three hours to complete the reaction and later cooled to room temperature. The contents were filtered using Whatman filter paper in order to remove any particulates formed during the reaction. The obtained stock solution is used for the deposition on to glass slides, quartz and silicon substrates by spin coating process [12]. Five sets of 25 glass slides precoated with nanoparticles by the above sol gel technique were taken into the study. Each set was pre coated with either pure TiO2 annealed at 200°C, or at 400°C, or TiO2 doped with 0.1%, 0.3%, 0.6% Silver (Ag).
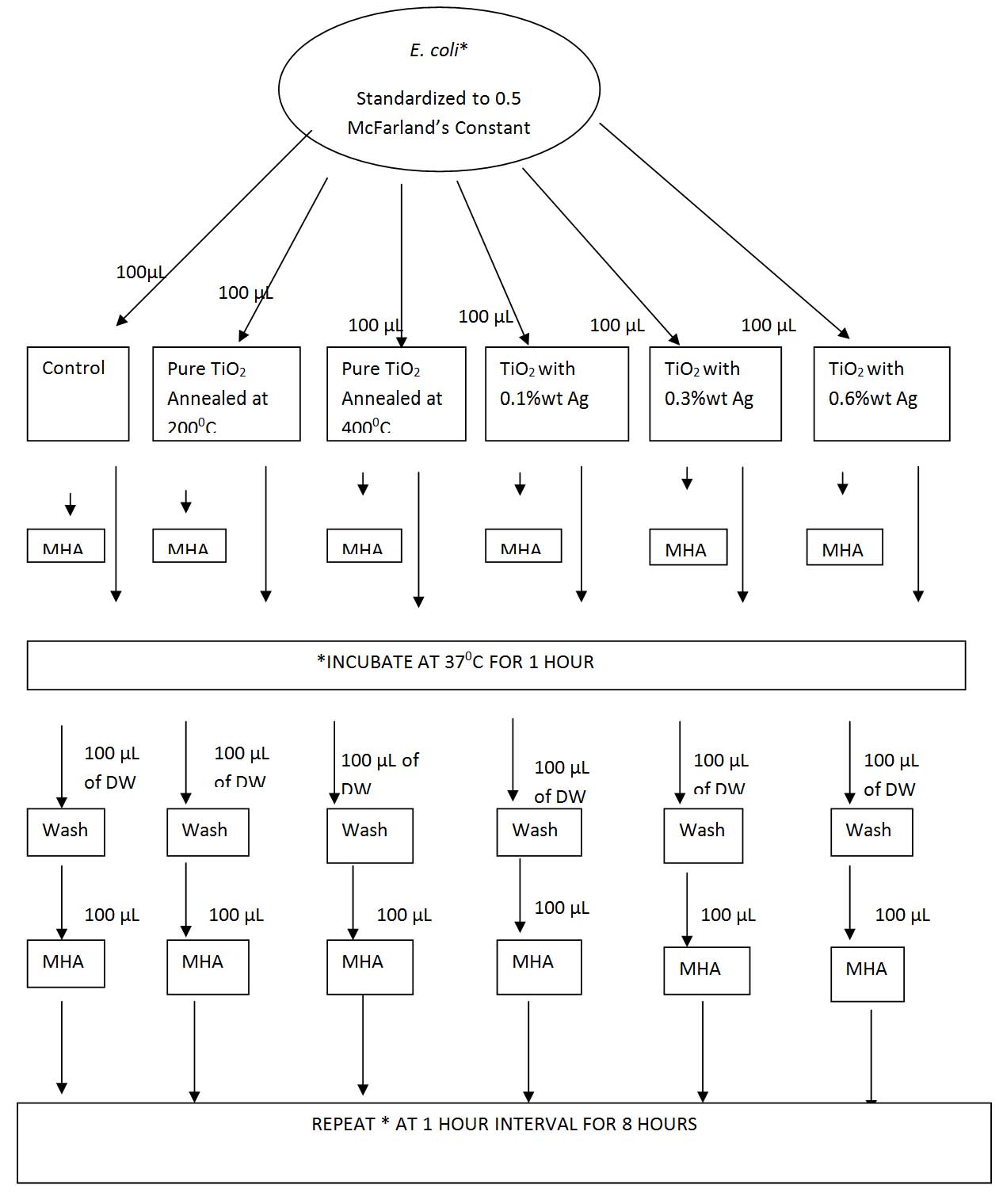
Antibacterial efficacy test: Each of the E. coli isolates was inoculated into peptone water and incubated at 37°C overnight. Next day, the turbidity was standardized to that of 0.5 McFarland standards. This was considered to be the working broth. Almost 100 μL of this broth was added onto one glass slide from each set, i.e., one coated with TiO2 annealed at 200°C, one annealed at 400°C, one each with TiO2 with 0.1% Ag as additive, TiO2 with 0.3% Ag, and TiO2 with 0.6% Ag as additive. A plain glass slide, not coated with any nanoparticle was used as a control. About 100 μL of the suspension was added this plain glass slide also. All these glass slides were incubated at 37°C in visible light. After one hour, all these suspensions are washed with 100 μL distilled water and 100 μL was taken from each of these emulsions and inoculated onto sterile Mueller Hinton Agar (MHA) plates. All these MHA plates were incubated in the incubator at 37°C overnight. The slides were placed back in the visible light. After every one hour interval for a total of eight hours, the same method was followed and each time the slides were washed with 100 μL distilled water and 100 μL of the washed suspension was inoculated onto fresh sterile MHA plates by streak method and incubated overnight in the incubator at 37°C. The Colony Forming Unit (CFU) was counted individually on a colony counter in each of these plates after overnight incubation [Table/Fig-1].
Flowchart of the inoculation procedure.
E. coli- Escherichia coli, TiO2- Titanium Oxide, Ag- Silver, MHA- Mueller Hinton Agar, DW – Distilled Water, μL – microliter
Results
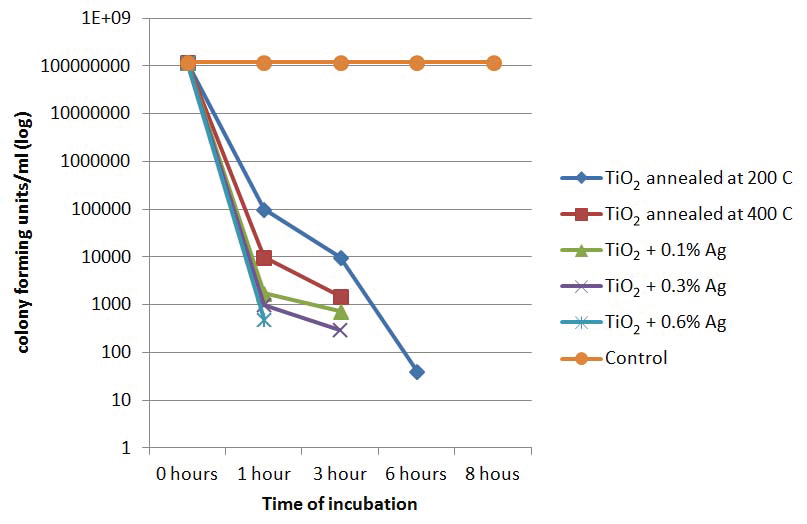
At the start of the experiment i.e., at 0 hours, all the 25 samples which were added to all the slides containing nanoparticles showed >1x108 cfu/ml. However, there was considerable reduction in the colony forming units on all the nanoparticle coated slides over time.
The organisms which were inoculated onto TiO2 annealed at 200°C showed a slower reduction rate, reducing from >1 x108 cfu/ml to <1x10 cfu/ml after six hours of incubation in visible light but the total absence of organisms was observed only after eight hours of incubation. However, in control slide, which was not treated with any of the nanoparticles, there was a growth of >1x108 cfu/ml even after 24 hours of incubation.
There was a considerable reduction in the cfu/ml in the TiO2 annealed at 400 °C from >1x108 cfu/ml to no growth after six hours of incubation, which was a slight improvement from theTiO2 annealed at 200°C. In plates with organism inoculated onto 0.1% Ag showed a decrease of cfu/ml to <1000 cfu/ml after four hours treatment and to no growth after six hours.
The organisms inoculated onto TiO2 coated slide with 0.3% Ag as additive showed a reduction of cfu/ml to <1x103 within three hours of growth itself which was faster than TiO2 with 0.1% Ag, while at the same time, there was no growth of any bacteria with TiO2 with 0.6% Ag as additive [Table/Fig-2]. At eight hours of incubation, except in the control plate, there was no growth in any of the other plates [Table/Fig-3]. [Table/Fig-4] shows that though TiO2 in its pure form was highly antibacterial, the antibacterial activity increased with the addition of silver and with the increase of the concentration of silver, this activity further increased.
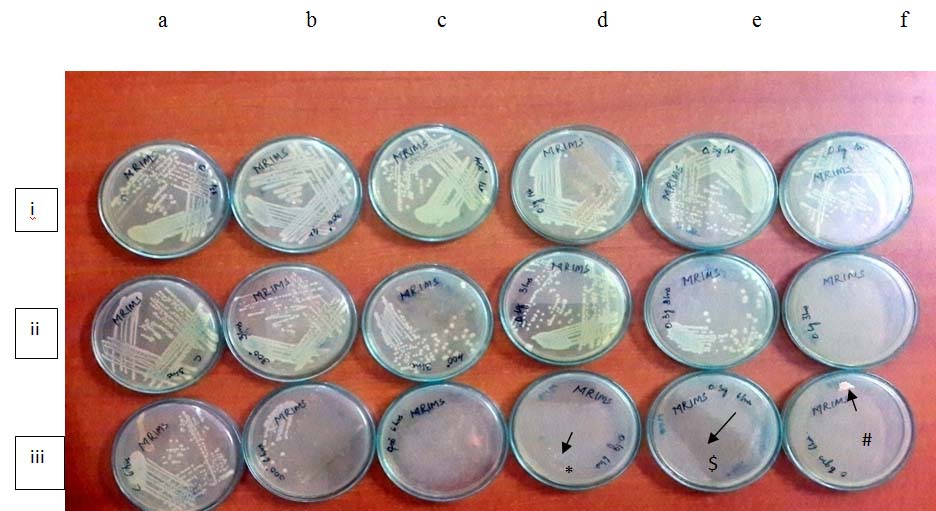
Growth of E. coli treated with TiO2 and TiO2 with silver as additives.
a: control, b: TiO2 annealed at 200°C, c: TiO2 annealed at 400°C, d. TiO2 with 0.1% Ag, e: TiO2 with 0.3% Ag, f; TiO2 with 0.6% Ag
i. At 0 hours of incubation
ii. At 3 hours of incubation
iii. At 6 hours of incubation
* and #: slight contamination in the media, $: shadow
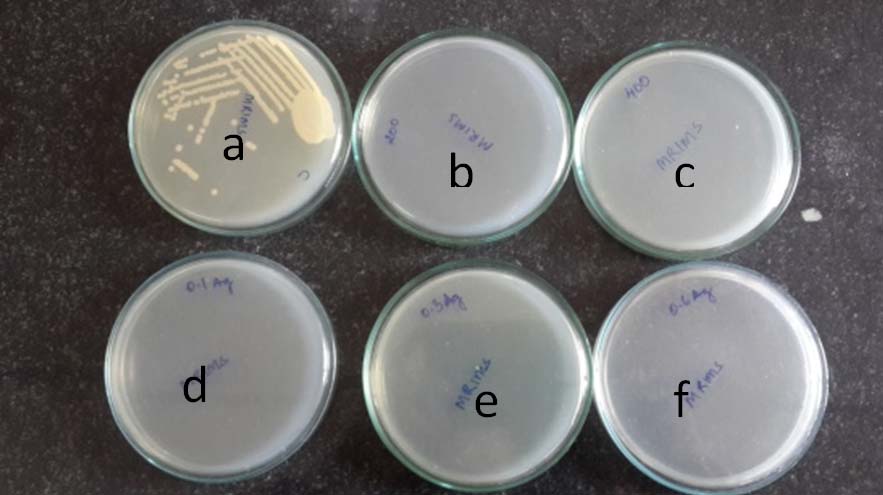
Growth of E. coli treated with TiO2 and TiO2 with silver as additives at 8 hours of incubation.
a: control, b: TiO2 annealed at 2000C, c: TiO2 annealed at 4000C, d. TiO2 with 0.1% Ag, e : TiO2 with 0.3% Ag, f; TiO2 with 0.6% Ag
Line graph showing rate of reduction of the cfu/mL of E. coli over time at different nanoparticle concentrations.
Discussion
The photocatalytic activity and the antibacterial properties of TiO2 have been improved by doping the surface with transitional metal ions and organic polymers. It is well demonstrated that the photocatalytic reactions are initiated by the most important trigger of light absorption that relates directly to the band gap between the valence and the conduction of TiO2 crystals. The photocatalytic reaction can be justified by the following processes. Firstly, light absorption takes place followed by excitation of electrons (with in nano seconds) from the valence band of TiO2 to the conduction band to form electron/hole pairs, this is preceded by migration of electron/hole pairs thus formed to the TiO2 surface. Secondly, initiation of, redox reactions take place with the hole of the valence reacting with H2O or hydroxide ions which are adsorbed on to the surface and lead to the formation of hydroxyl radicals as well as superoxide ions. These hydroxyl ions are very powerful and are indiscriminate oxidizing agents against the bacteria [16].
It has been suggested by Zhang Y et al., that the metal oxides consist of a positive charge while the microorganisms exhibit a negative charge, which is the reason of the electromagnetic effect between the organisms and the metals, leading to the oxidation of the organisms, ultimately resulting in the death [17]. However, Sangchay W et al., in his study has reported that be the photocatalytic effect of TiO2 causing the hydroxyl radical attack and lipid peroxidation reaction. The silver ions increase the oxygen anion radicals and as a result causing reactive centre in the TiO2 surface. With water, this photogenerated holes can react and form hydroxyl radicals [18].
In the present study, we have observed the antibacterial effect of pure TiO2 annealed at 200°C and 400°C and TiO2 doped with 0.1% Ag, 0.3% Ag and 0.6% Ag on drug sensitive E. coli when incubated in visible light. Our study showed a steady decrease of colony forming units/ml from 0 hours to eight hours. At 0 hours, as the stock solution was adjusted to 0.5 McFarland’s constant, all the plates had a growth of around 1.5x108 cfu/ml. At three hours, there was a steady decrease to 103 cfu/ml in all the conditions. By eight hour interval, there was no cfu/ml on all the nanoparticle coated glass slide while the one on control slide showed confluent growth (>1x108 cfu/ml). In the TiO2 annealed at 200°C, the number of colony forming units reduced to <1x100 cfu/ml at sixth hour interval while in TiO2 annealed at 400°C, there was no growth at sixth hour interval. However, in the slide containing TiO2 doped with 0.6% wt Ag, there was a slight contamination due to mishandling of the slides at sixth hour interval [Table/Fig-2].
In a study by Sangchay W et al., silver doped TiO2 thin films eliminated E. coli much faster than TiO2 alone even under UV radiation [19]. These results were in accordance to our study, where TiO2 doped with Ag were much more effective than only pure TiO2. With increasing concentrations of silver, the antibacterial effect was faster. In a similar study by Sophee S et al., a reduction of the colony forming units/ml in the ATCC strains of E. coli when treated with TiO2 with increased concentrations of zinc oxide as the additive. Similar results were observed when incubated both in visible and UV light showing that zinc oxide also had similar antibacterial effect as silver [20].
Efficacy of TiO2 as a topical agent as well as in combination therapy was analyzed by other researchers in their study and reported that an increase in the antibacterial effect was associated with the increase in the concentration of TiO2 [21].
Silver as additive for antibacterial effect was also tested on other ATCC strains of bacterial pathogens such as Staphylococcus aureus and Staphylococcus epidermidis, with similar results [12]. Several investigators have reported oxidative damage to cell membrane or internal cell structures with or without illumination [20,22].
With the emergence of multiresistant bacteria, there is an urgent need for newer techniques that can efficiently kill them. Nanoparticles have offered a new strategy to tackle these bacteria. It has been reported that four types of silver carbon complexes with different formulations are effective against many such medically important bacteria such as Pseudomonas, Acinetobacter, Methicillin Resistant Staphylococcus aureus (MRSA) [3].
Most of the studies studied earlier were done on water samples or on ATCC strains [12,18-22]. The present study was one of the very few studies which used actual clinical samples and human pathogens instead of ATCC strains. It was observed that the antibacterial effect of the nanoparticles on clinical samples was similar to that on ATCC strains. This study has opened the path to venture the efficacy of the nanoparticle coated implants on the infectious human pathogens. Future studies need to be performed in vivo to see the effects of nanoparticles of TiO2 with or without Ag within the body after the implantation of the biomedical device.
Limitation
However, there are few limitations of the present study. This study was performed only on non Extended Spectrum of Beta Lactamases (ESBL) Escherichia coli isolated from the clinical samples. The sample size was small with only 25 samples of E. coli. Further tests need to be performed on multi drug resistant E. coli such as ESBLs as well as on other coliforms and bacterial pathogens. There are other additives such as copper, zinc that can be added to TiO2. Comparison of the efficacy of antibacterial effect of silver and the other additives can also be performed.
Conclusion
TiO2 is found to be antibacterial and thus can be used as disinfectant. When doped with silver, the antibacterial effect was increased. Thus, efficiency of nanoparticles as biocide agent can be increased doping with some other metal oxides. The research on biomedical applications of nanoparticles has only recently begun. Further research can be conducted by coating nanoparticles onto biomedical appliances such as scalpels and other implants to assess their efficacy in vivo.
[1]. Burke JP, Infection control—a problem for patient safetyN Engl J Med 2003 348:6516 [Google Scholar]
[2]. Nichols RL, Preventing surgical site infections: a surgeon’s perspectiveEmerg Infect Dis 2001 7(2):220-24. [Google Scholar]
[3]. Liu C, Zhao Q, Liu Y, Wang S, Abel EW, Reduction of bacterial adhesion on modified DLC coatingsColloids Surf B Biointerfaces 2007 15;61(2):182-87. [Google Scholar]
[4]. Bosettia M, Masse A, Tobinc E, Cannas M, Silver coated materials for external fixation devices: in vitrobiocompatibility and genotoxicityBiomaterials 2002 23:887-90. [Google Scholar]
[5]. Wassail MA, Santin M, Isalberti C, Cannas M, Denyer SP, Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pinsJ Biomed Mater Res 1997 36(3):325 [Google Scholar]
[6]. Olorunmola FO, Kolawole DO, Lamikanra A, Antibiotic resistance and virulence properties in escherichia coli strains from cases of urinary tract infectionsAfr J Infect Dis 2013 7(1):1-7. [Google Scholar]
[7]. Katsikogianni M, Missirlis YF, Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactionsEur Cell Mater 2004 7;8:37-57. [Google Scholar]
[8]. Buzea C, Pacheco II, Robbie K, Nanomaterials and nanoparticles: sources and toxicityBiointerphases 2007 2(4):MR17-71. [Google Scholar]
[9]. Kiwi J, Nadtochenko V, Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopyLangmuir 2005 21:4631-41. [Google Scholar]
[10]. Matsunaga T, Tomada R, Nakajima T, Wake H, Photochemical sterilization of microbial cells by semiconductor powdersFEMS Microbiology. Letters 1985 29:211-14. [Google Scholar]
[11]. Gogniat G, Kan D S, TiO2 photocatalysis causes DNA damage via fenton reaction-generated hydroxyl radicals during the recovery periodAppl Environ Microbiol 2007 73:740-43. [Google Scholar]
[12]. Prasad RGSV, Basavaraju D, Rao KN, Shivappa NC, Endrino JL, Phani AR, Nanostructured TiO2 and TiO2-Ag antimicrobial thin filmsNanoscience, Technology and Societal Implications (NSTSI) 2011 :1-6. [Google Scholar]
[13]. Foster HA, Ditta IB, Varghese S, Steele A, Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activityAppl Microbiol Biotechnol 2011 90:1847-68. [Google Scholar]
[14]. Klasen HJ, A historical review of the use of silver in the treatment of burns. II. Renewed interest for silverBurns 2000 26(2):131-38. [Google Scholar]
[15]. Adams AP, Santschi EM, Mellencamp MA, Antibacterial properties of a silver chloride-coated nylon wound dressingVet Surg 1999 28(4):219-25. [Google Scholar]
[16]. Palza H, Antimicrobial polymers with metal nanoparticlesInt J Mol Sci 2015 16:2099-2116. [Google Scholar]
[17]. Zhang Y, Kohler N, Zhang M, Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptakeBiomaterials 2002 23:1553-61. [Google Scholar]
[18]. Sangchay W, The self-cleaning and photocatalytic properties of TiO2 doped with SnO2 thin films preparation by sol-gel methodEnergy Procedia 2016 89:170-76. [Google Scholar]
[19]. Sangchay W, Rattanakun T, Photocatalytic, Antibacterial and Self-Cleaning Properties of TiO2 and Ag Doped TiO2 Thin Films Using Sol-Gel MethodRMUTP Research Journal Special Issue The 4thRajamangala University of Technology International Conference [Google Scholar]
[20]. Sophee S, Prasad RGSV, Srinivas JV, Aparna RSL, Phani AR, Antibacterial activity of TiO2 and ZnO microparticles combination on water polluting bacteriaJournal of Green Science and Technology 2012 1:1-7. [Google Scholar]
[21]. Ahmad R, Sardar M, TiO2 nanoparticles as an antibacterial agent against E. coliInternational Journal Of Innovative Research In Science, Engineering And Technology 2013 2(8):3569-74. [Google Scholar]
[22]. Prasad RGSV, Endrino JL, Jain NK, Reddy G, Basavaraju D, Rao KN, Synthesis and antimicrobial properties of TiO2 and TiO2: Cu thin films synthesised by a cost effective sol–gel processAdv Sci Eng Med 2012 4:1-9. [Google Scholar]