The past decade has seen tremendous development in the wireless telecommunications industry with the introduction of cellular phones (cell phones/mobile phones). The development has revolutionized the telecom industry by making telecommunication faster, economical and more convenient [1]. With the introduction of new applications and multifunctional features in the mobile hand set, the telecom industry is wooing both young and old generation.
Pulsed electromagnetic radiations are created when the cell phone is in operation [2] most of which are absorbed by body surface particularly the head region. This absorbed energy causes both thermal and non-thermal stress in the body [3,4]. Non-thermal stress is more deleterious than thermal stress and is known to cause oxidative stress [5], production of free radicals [6], structural changes in plasma membrane [7], changes in ionic transport [8] and also increased DNA damage [9].
Contradictory reports are available regarding the structural changes in various tissues like retina, liver, kidney and testis in different animal models on exposure to electromagnetic radiation of 900- 1800 MHz. The exposure resulted in impaired retinal growth, malformed brain [10] hyperpigmentation of Retinal Pigment Epithelium (RPE) [11] structural changes in the retina [12] and lens [1]. Nevertheless, several studies showed no histopathological changes in the various ocular tissues [13,14]. Structural changes were also observed in the kidney [15] and liver [16-18] of different animal models on RFR exposure. However, Rajaei F et al., reported no significant structural changes in liver on exposing mice models to electromagnetic fields of 50 MHz [19].
Contradictory reports are available on the role of RFR in causing oxidative stress in various biological tissues of different animal models [20-22]. A long term exposure of albino Wistar rats to RFR from 900 MHz mobile phones resulted in decreased concentration of antioxidant enzyme Glutathione Peroxidase (GPx), catalase and Superoxide Dismutase (SOD) [20] and increased Reactive Oxygen Species (ROS) by means of Fenton reaction [21]. However, no significant change in the antioxidant activities was observed in albino Wistar rats on exposure to RFR emitted from 3G mobile phone [22].
Several scientific reports are available to establish the role of RFR emitted from cell phones in causing DNA damage in various biological tissues in both human and animal models [23-26]. The long term exposure resulted in increased DNA strand breaks, Single Strand Breaks (SSB’s) and Double Strand Breaks (DSB’s), and rearrangement of DNA segments in testis [23], brain [24] and eyes [25] of different animal models and also in human lymphocyte culture [26]. However, various authors reported no significant DNA damage in different tissues of various animal models [27] and in human lymphocyte culture [28].
The cell phone once considered as a status symbol in early 90’s has now become an integral part of everyone’s life. They are used extensively by all age groups, including the children, elderly and pregnant women. Researches involving children and pregnant women are very less due to various ethical issues. The present study may provide an insight into basic mechanisms by which RF fields interact with developing tissues in an embryo. We have designed this study to evaluate the possible effects of chronic exposure to RFR emitted from 2G and 3G mobile phones on developing chick embryo liver.
Materials and Methods
The present work was an experimental study that has been carried out during the year 2011-2015. All the procedures were followed as per Ethical Guidelines for care and use of experimental animals approved by Institutional Animal Ethical Committee (IAEC). Fresh fertile hen eggs (Gallus domesticus) having approximately similar weight (65-70 gm±5 gm) was procured from Rajiv Gandhi College of Veterinary and Animal Sciences, Puducherry, India. The eggs were incubated in 19 batches of 12 eggs each (total-228±20 eggs) in a standard egg incubator at 37±0.5°C and 50%-55% of humidity and ventilation.
The first four batches (48 eggs) were grouped as control (Group–D) and they were incubated without any external factors interfering with their developmental process. Next five batches (60 eggs) were treated as sham exposed group (Group-C). They were incubated along with a popular brand cell phone with the SAR of 0.310 watts/kilogram hung from above with 5 cm distance separating the egg and kept in null status (switched off). Morphological features and structure of liver of both these groups were analysed and found to be similar. So, we have considered the sham exposed group as the control group for the present study.
The experimental group, Group–A (exposed to 2G cell phone radiation) and Group–B (exposed to 3G cell phone radiation), were also incubated (60+60 eggs) in a similar manner with the cell phone kept in silent operative mode with head phone plugged in (switched on). This arrangement ensured that the cell phone got activated automatically each time it received a call and the intensity of radio frequency waves were measured using radiofrequency meter (RF meter, Less EMF Inc, USA) [Table/Fig-1].
A photograph showing the experimental set up. The Mobile phone (red arrow) is hung with a distance of 5 cm separating it from the fertilized chicken eggs. A RF meter is kept inside the incubator to check the intensity of radiation (yellow arrow).

A popular brand cell phone hand set and a service provider were used for network connection for both 2G and 3G exposure. For exposure activation, the cell phone was rung from another cell phone for duration of three minutes each, every half an hour, with the first exposure given at 12th hour of incubation (4.30 am-4.30 pm). The total exposure for a 12 hour period was 75 minutes followed by 12 hour of exposure-free period. This was repeated daily up to 12th day of incubation.
Six embryos per day were terminated from 5th day to 12th day. The embryos were carefully transferred to a petridish containing normal saline and the various growth parameters (weight, volume and CR length) were recorded. The embryos were then fixed in 10% formalin and processed for routine histological studies. Five micron thick sections were cut in sagittal plane, coronal plane and in transverse plane and stained with H&E and PAS stain. Degenerative changes in hepatocytes were observed and compared in all groups. The nuclear diameter of hepatocytes was measured from a randomly selected field of every fifth section of a slide using calibrated oculometer under oil immersion. The healthy nuclei showing prominent nucleoli were used for the measurement. The number of nuclei showing karyorrhexia (nuclear fragmentation) was counted in a randomly selected field using square reticule under oil immersion and the values obtained were statistically analysed using one-way ANOVA using Graph Pad Instat 3.
The liver of fifth batch of embryos of all the three groups (12+12+12) were subjected to alkaline comet assay technique developed by Singh NP [29] with modifications in staining procedure [30], for assessing the DNA damage. The slides were stained with silver nitrate and then analysed using automated comet scoring software (Comet Score IV) to assess and quantify the levels of DNA damage in three groups. The mean comet length, the mean tail length, mean % of DNA in the tail and mean tail moment of all three groups were statistically compared using one-way ANOVA with Graph Pad Instat 3.
Results
The growth parameters like volume, weight and CR length of exposed groups showed significant changes in comparison with the control group. The growth parameters were increased in 2G exposure group than control group. The 3G exposure resulted in decreased growth parameters in correlation with control and 2G exposure groups. However, by 11th and 12th day all the three growth parameters became more or less equal in all the three groups [Table/Fig-2,3 and 4].
Mean volume of chick embryos in all the three groups.
| Age indays | Control group(Group-C) (in cc) | 2G group(Group-A) (in cc) | 3G group(Group- B) (in cc) |
|---|
| 5 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.008 |
| 6 | 0.3 ± 0 | 0.3 ± 0 | 0.18 ± 0.01*** |
| 7 | 0.53 ± 0.03 | 0.8 ± 0.08** | 0.5 ± 0** |
| 8 | 1 ± 0 | 1.02 ± 0.025 | 1 ± 0 |
| 9 | 1.21 ± 0.09 | 1.76 ± 0.13 | 1 ± 0.20* |
| 10 | 1.5 ± 0 | 2.35 ± 0.16** | 1.6 ± 0.16** |
| 11 | 2.16 ± 0.10 | 2.5 ± 0.12 | 2.2 ± 0.12 |
| 12 | 3.41 ± 0.15 | 3.25 ± 0.17 | 3.3 ± 0.12 |
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis < 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Mean weight of chick embryos in all the three groups.
| Age indays | Control group(Group-C)(in gm) | 2G group(Group-A)(in gm) | 3G group(Group- B)(in gm) |
|---|
| 5 | 0.143 ± 0.007 | 0.129 ± 0.01 | 0.087 ± 0.002** |
| 6 | 0.298 ± 0.01 | 0.316 ± 0.007 | 0.216 ± 0.01** |
| 7 | 0.613 ± 0.009 | 0.738 ± 0.05* | 0.582 ± 0.03 |
| 8 | 0.864 ± 0.01 | 1.098 ± 0.02*** | 0.945 ± 0.03 |
| 9 | 1.290 ± 0.04 | 1.643 ± 0.15 | 1.066 ± 0.20 |
| 10 | 1.468 ± 0.01 | 2.239 ± 0.11*** | 1.837 ± 0.09* |
| 11 | 2.189 ± 0.04 | 2.477 ± 0.08 | 2.146 ± 0.16 |
| 12 | 3.433 ± 0.11 | 3.200 ± 0.14 | 3.345 ± 0.11 |
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis < 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Mean CR length of chick embryos in all the three groups.
| Age indays | Control group(Group-C) (in mm) | 2G group(Group-A) (in mm) | 3G group(Group- B) (in mm) |
|---|
| 5 | 11 ± 0.31 | 8.98 ± 2.04 | 11 ± 0.25 |
| 6 | 13.6 ± 0.4 | 14 ± 0 | 12.3 ± 0.49 |
| 7 | 19.16 ± 0.40 | 18.75 ± 0.62 | 16.75 ± 0.25** |
| 8 | 21.12 ± 0.31 | 22.5 ± 0.28 | 20.16 ± 0.40 |
| 9 | 23.41 ± 0.41 | 24.33 ± 1.2 | 22.37 ± 1.4 |
| 10 | 25.16 ± 0.87 | 28 ± 0.44* | 27.33 ± 0.49 |
| 11 | 29 ± 0.73 | 31.5 ± 0.5 | 30 ± 1.51 |
| 12 | 35.16 ± 0.54 | 34.16 ± 1.4 | 34.8 ± 0.37 |
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
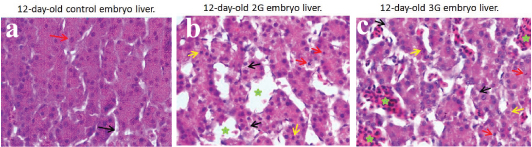
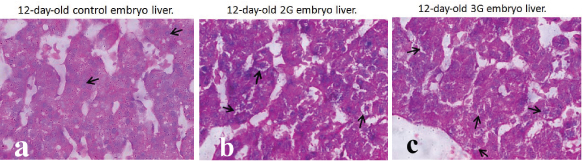
Histological evaluation of control embryo liver in all the age groups (5th–12th day) showed normal architecture with hepatocytes arranged in the form of hepatic cords and sinusoids separating them. The sinusoids showed mild to moderate numbers of RBC’s. The hepatocytes showed eosinophilic granular cytoplasm and centrally placed large vesicular nuclei with prominent nucleoli. The cytoplasmic changes in the form of vacuolations and nuclear changes (karyorrhexis, pyknosis) were indistinct in the control group.
The liver structure in 2G and 3G experimental groups (5th day- 12th day) showed marked dilation of sinusoidal spaces (peliosis hepatis) with moderate to more numbers of RBC’s indicating haemorrhage in the parenchyma. The cytoplasm of hepatocytes showed vacuolations rendering them foamy appearance. These vacuolations were PAS negative that indicated fatty changes (steatosis). The nuclei showed karyorrhexis and pyknotic changes [Table/Fig-5,6].
a) Photomicrograph of 12-day-old control embryo liver showing normal looking hepatocytes with eosinophilic cytoplasm and large vesicular nuclei (red arrow) and normal hepatic sinusoids (black arrow) (H&E x 1000); b,c)Photomicrograph of 12-day-old 2G and 3G embryo liver showing hepatocytes with vacuolations in the cytoplasm (black arrow), pyknotic nuclei (red arrow), karyorrhexis (yellow arrow) and dilatedhepatic sinusoids with RBCs (green asterix) (H&E x 1000).
a) Photomicrograph of 12-day-old control embryo liver showing normal looking hepatocytes in hepatic cords (black arrow) (PAS x 1000); b,c) Photomicrograph of 12-day-old 2G and 3G embryo liver showing vacuolations in the cytoplasm of hepatocytes (black arrow) (PAS x 1000).
The nuclear diameter of hepatocytes in all the three groups showed a gradual increase in diameter as the age advanced. The nuclear changes observed in our study were swollen appearance (nucleomegaly) of hepatic nuclei in 2G and 3G group embryos. Both the 2G and 3G exposed groups showed increased nuclear diameter than the control group which was significant on all days (p<0.001). On comparing 2G and 3G groups, 3G group embryos showed increased diameter and was significant on all days [Table/Fig-7].
Mean nuclear diameter of hepatocytes in all the three groups of chick embryo.
| Age indays | Control group(Group-C) (in mm) | 2G group(Group-A) (in mm) | 3G group(Group- B) (in mm) |
|---|
| 5 | 0.0039 ± 0.00002 | 0.0041 ± 0.00003** | 0.0042 ± 0.00004*** |
| 6 | 0.0039 ± 0.00002 | 0.0042 ± 0.00004*** | 0.0043 ± 0.00004*** |
| 7 | 0.0040 ± 0.00002 | 0.0042 ± 0.00003*** | 0.0043 ± 0.00004*** |
| 8 | 0.0040 ± 0.00001 | 0.0042 ± 0.00004*** | 0.0044 ± 0.00004*** |
| 9 | 0.0041 ± 0.00002 | 0.0043 ± 0.00004*** | 0.0045 ± 0.00004*** |
| 10 | 0.0041 ± 0.00003 | 0.0044 ± 0.00004** | 0.0045 ± 0.00005*** |
| 11 | 0.0042 ± 0.00004 | 0.0044 ± 0.00004* | 0.0047 ± 0.00004*** |
| 12 | 0.0043 ± 0.00004 | 0.0046 ± 0.00004*** | 0.0049 ± 0.00004*** |
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Both 2G and 3G group embryos showed increased karyorrhexis than the control group embryos. The increase was significant on 5th and 12th day for 2G group embryos (p<0.01) and for 3G group embryos the increase was significant on 5th, 7th, 9th, 11th and 12th day of incubation (p<0.001, p<0.01, p<0.01, p<0.001 and p<0.001 respectively). On comparing between 2G and 3G group, 3G group showed increased karyorrhexis than 2G group but the increase was significant only on 5th, 7th, 9th and 11th day (p<0.05, p<0.05, p<0.05 and p<0.001 respectively) [Table/Fig-8].
Karyorrhexis in nuclei of hepatocytes in all the three groups of chick embryo.
| Age indays | Control group(Group-C) | 2G group(Group-A) | 3G group(Group- B) |
|---|
| 5 | 0.84 ± 0.08 | 1.42 ± 0.13** | 1.5 ± 0.12*** |
| 6 | 1.14 ± 0.09 | 1.32 ± 0.13 | 1.5 ± 0.13 |
| 7 | 1.06 ± 0.16 | 1.22 ± 0.14 | 1.72 ± 0.12** |
| 8 | 1.5 ± 0.14 | 1.5 ± 0.10 | 1.92 ± 0.16 |
| 9 | 1.1 ± 0.11 | 1.3 ± 0.11 | 1.74 ± 0.13** |
| 10 | 1.21 ± 0.11 | 1.22 ± 0.11 | 1.38 ± 0.13 |
| 11 | 1.27 ± 0.10 | 1.32 ± 0.09 | 2.02 ± 0.16*** |
| 12 | 0.72 ± 0.13 | 1.32 ± 0.11** | 1.62 ± 0.10*** |
Values are means ± SEM taken from 6 samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant
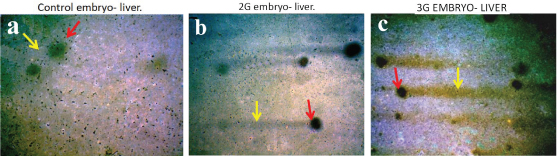
On assessing the DNA damage in the form of DSB’s, we found significant increase in all the four parameters of both 2G and 3G group embryos in all the days (9th – 12th day) in comparison with the control group embryos (p<0.001 and p<0.001 respectively) [Table/Fig-9]. On comparing between 2G and 3G group embryos, it was found that the 3G group embryos showed significant increase in all the four parameters than the 2G group embryos [Table/Fig-10].
Estimation of DNA damage in liver in all the three groups (9-12 days).
| Age indays | Mean cometlength (μm) | Mean taillength (μm) | Mean % ofDNA in tail (μm) | Mean tailmoment (μm) |
|---|
| 9 (CON) | 4.03 ± 0.15 | 4.18 ± 0.1 | 21.63 ± 0.6 | 80.55 ± 3.0 |
| 9 (2G) | 7.9 ± 0.18** | 6.06 ± 0.1*** | 31.76 ± 1.6*** | 184.89 ± 6.2*** |
| 9 (3G) | 7.8 ± 0.22*** | 6.12 ± 0.2*** | 47.16 ± 1.7*** | 258.69 ± 11.5*** |
| 10(CON) | 4.6 ± 0.19 | 3.90 ± 0.1 | 21.23 ± 0.5 | 84.49 ± 3.1 |
| 10(2G) | 7.3 ± 0.14*** | 6.40 ± 0.2*** | 34.05 ± 1.3*** | 157.13 ± 5.8*** |
| 10(3G) | 7.4 ± 0.15*** | 6.39 ± 0.2*** | 48.07 ± 1.4*** | 260.53 ± 12.3*** |
| 11(CON) | 5.2 ± 0.18 | 3.70 ± 0.1 | 22.46 ± 0.6 | 92.48 ± 3.0 |
| 11(2G) | 8.3 ± 0.24*** | 6.35 ± 0.2*** | 42.43 ± 1.2*** | 240.54 ± 15.1*** |
| 11(3G) | 9.5 ± 0.14*** | 8.30 ± 0.2*** | 48.94 ± 1.5*** | 292.16 ± 11.6*** |
| 12(CON) | 6.0 ± 0.13 | 4.38 ± 0.1 | 23.10 ± 0.6 | 93.99 ± 3.2 |
| 12(2G) | 8.9 ± 0.17*** | 7.15 ± 0.1*** | 47.14 ± 1.8*** | 248.08 ± 14.1*** |
| 12(3G) | 9.8 ± 0.15*** | 8.24 ± 0.2*** | 49.67 ± 1.5*** | 298.69 ± 15.3*** |
Values are means ± SEM taken from 3 samples per day for control, 2G and 3G group (n = 36 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
a) Photomicrograph of 12-day-old control embryo comets showing minimal DNA damage. Head diameter is large (red arrow) and tail length is shorter (yellow arrow) 10X; b,c) Photomicrograph of 12-day-old 2G and 3G embryo comets showing severe DNA damage. Head diameter is decreased (red arrow) and tail length is increased (yellow arrow) 10X.
Discussion
In the present study, increased growth parameters of 2G group embryos could be due to increased cellular proliferation coupled with Ca2+ influx into the cells on exposure to RFR [31]. Similar observation was reported earlier by Fatima Al Qudsi et al. In their study, the exposure of chick embryos (900-1800 MHz) increased the growth parameters (the CR length, body length and beak length) and also increased the eye development (eye weight, eye diameter, eye area, eye perimeter) on the tenth day of incubation and a decrease in these parameters was observed on further exposure [10].
However, the decreased growth parameters for 3G group embryos in the present study, probably could be due to RFR interactions at cellular level (free radical production) and molecular level (DNA damage) resulting in genotoxicity. This in turn might affect cell proliferation either by increasing or reducing the proliferation rate and thus plays an important role during early embryonic development [31,32]. A number of studies are available reporting retarded growth on exposure to RFR of similar frequency [12,33,34]. Decreased foetal weight and crown-rump length was observed in intrauterine exposure of rat and mouse animal models to RFR ranging from 27.12 MHz to 2450 MHz [12].
The similar growth parameters observed in all the three groups of embryos on 11th and 12th day probably could be due to difference in cellular responses to RFR at different embryological periods and the cells might be trying to rebalance their growth and differentiation rate to normal by activating various cellular stress response mechanisms [10].
In the present study, the cytoplasmic vacuolations observed in hepatocytes of 2G and 3G group embryos were similar to the observations reported by different researchers. The exposure of white leghorn chicken embryos to 50 Hz electromagnetic fields showed fibrotic bands in hepatocytes, severe steatohepatitis, degenerated hepatocytes, abnormal lipid accumulation and lipid droplets pushing hepatocytes nuclei to the corner of the cell [16]. The exposure of pregnant albino rats to 900 MHz for 1 hour per day from 13th to 21st day of pregnancy resulted in histopathological changes in the liver of 21 day old neonatal rats. The changes observed were necrotic hepatocytes with hydropic changes in the liver parenchyma especially near peri-central area and irregular nuclei [17]. Tarantino P et al., reported vacuolations in cytoplasm and presence of granules in hepatocytes of rabbit liver on exposure to 650 MHz electromagnetic fields for 18 months continuously [18]. However, in the present study necrotic changes were not observed. In one of the studies, the exposure of mice to electromagnetic fields of 50 MHz and intensity of 0.5 mT 4 hours per day for two months showed no significant increase in necrotic cells and kuffer cells and no structural changes in liver [19]. Khalil A et al., exposed Balb/c mice to RFR of 900 MHz for 30 minutes/day for one month. Their study showed no significant histopathological changes in different tissues including liver [35].
In our study, the dilated sinusoidal spaces of both 2G and 3G group embryos might probably be due to endothelial injury caused by RFR that would have allowed blood to accumulate in spaces of Disse with resultant formation of the cavities [36]. Sinusoidal dilatations in hepatic parenchyma have been reported earlier by other researchers. The exposures of young male rats to electromagnetic radiations lead to sinusoidal dilatation in the parenchyma and periportal area of liver tissue [37]. Sinusoidal expansion and irregular sinusoidal lumen diameter was observed in rabbit liver on exposure to 650 MHz radiation for 12 and 18 months respectively. Moreover, infiltrations within the sinusoids of chick embryo liver were also reported by Lahijani MS et al., [16]. In our study also sinusoids showed infiltrations and were engorged with RBCs.
The nucleomegaly observed in the hepatocytes of 2G and 3G group embryos of the present study might be due to hydropic changes/oncosis triggered by RFR exposure. Glycogenated nuclei or hydropic nuclei could be encountered in various pathological conditions [36]. Previous studies have reported the presence of dentated nuclei in chick embryo liver on exposure to 50 Hz electromagnetic radiations [16] and necrotic hepatocytes with irregular nuclei [17]. In the present study, the increased nuclear diameter and karyorrhexis observed in the 3G group embryos, on comparing with 2G and control group embryos, might be due to increased lethality of 3G radiations.
RFR emitted from cell phone contains electric and magnetic energy moving together in space [38]. RFR enhances the production of free radicals and its magnetic component could make the free radical stay for longer time, thus, giving them the potential to do more damage. The high levels of free radicals could overwhelm the cellular antioxidant capacity and above a certain threshold level could result in cellular damage. The free radicals if added to unsaturated cell membrane lipids can damage membrane lipids thus changing the integrity of membrane. This could lead to influx of water, Na+ and Ca2+ ions causing swelling and vacuolization of the cytoplasm (cytomegaly), nuclear swelling (nucleomegaly), rupture of nucleus and nucleolus, pyknosis, karyorrhexis, karyolysis and cytolysis [39].
In the present study, the increased DNA damage observed in 2G and 3G group embryos were in correlation with the previous studies. Increased DNA damage was observed in the liver cells of Wistar rats on exposure to 915 MHz radiations with a power density of 2.4 W/m2 and SAR of 0.6 W/kg one hour per day, seven days per week for two weeks [40]. In our study, the increased parameters showed by 3G group embryos on comparing with 2G group embryos, indicates their increased lethality.
DNA are remarkably stable macromolecules that controls growth and function of cells. Researchers have postulated different mechanisms by which an EMF interacts with DNA causing damages. The energy associated with RFR cannot directly break the chemical bonds within the molecules. However, it could enhance the free radical activity especially hydroxyl ions (HO•) in the cells by Fenton reaction. HO• thus produced are unique in that they get added to DNA very rapidly in vivo yielding altered bases or SSB and DSB [39]. Another possible mechanism is that, RF radiation is known to produce alterations in the structure of proteins [41]. Thus, structural alteration in DNA repair enzymes might have caused changes in its function, leading to DNA damage [42].
Limitation
The direct extrapolation of the outcome of the present study to human population may be limited due to differences in species, volume and size, life span, functional and anatomical organization of tissues. Further research is warranted to enhance better understanding of underlying principles of RFR interaction with biological tissues.
Conclusion
From our experimental outcome, we conclude that the chronic exposure of chick embryo liver to RFR emitted from 2G and 3G cell phone resulted in various structural changes and DNA damage. The changes were more pronounced in 3G experimental group. Many researchers now opine that cell phones may turn out to be the cigarettes of 21st century as their effects or interactions with biological tissues on long term exposure are yet to be explored especially in foetuses and children. Hence, children and pregnant women should use the cell phone with caution. Introduction of new generation phones, 4G and 5G, open a vast potential for future research and whether these changes observed due to RFR exposure are reversible or not on withdrawing the exposure is another arena which warrants further research.
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis < 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis < 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Values are means ± SEM taken from six samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)
Values are means ± SEM taken from 6 samples per day for control, 2G and 3G group (n = 48 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant
Values are means ± SEM taken from 3 samples per day for control, 2G and 3G group (n = 36 chick embryos) (analysis< 0.05* significant, < 0.01** highly significant and < 0.001 *** extremely significant)