OLP first described by British Physician Wilson Erasmus in 1869, is a common immunologically mediated mucocutaneous disorder. It has a diverse pattern of manifesting itself clinically which may range from affecting just the skin to oral and genital mucosa, nails and also the scalp sometimes [1]. A dental surgeon is one among the first people who is presented with a chance to identify this lesion due to the commonality with which it occurs in the oral mucosa. The onus of responsibility to aid early identification and diagnose this lesion lies entirely with the Oral Pathologist who can aid both the practitioner and the patient by serving them both right.
Throughout its history spanning over one and a half centuries almost, what is clear to an extent is the number of possible etiologic factors and an autoimmune pathogenic mechanism which leads to formation of OLP. However, an intriguing aspect of OLP is its malignant potential. Much has been written but very little has actually been substantiated with regards to this detrimental change seen sometimes in OLP. Ever since it was first written about by Hallopeu [2] in 1910, up until the WHO officially decided to term OLP as a potentially malignant disorder, a lot of controversy has always surrounded it. Inspite of all this, there is still no definite malignant mechanism attributed to OLP. The current hypothesis banks on the chronic inflammatory component seen almost always in the underlying stromal component in OLP exerting two varied signals; one on the overlying epithelium and at the same time another on the stroma itself to convert it into a tumour-like environment which will aid in malignant changes [3].
OLP is known to make the underlying stroma hypoxic, with concentration of oxidative stresses present within and adjacent to the basal cell layer [4,5], that would result in the upregulation of proteases in the extracelluar matrix as well as in epithelial cells. These would facilitate spread, invasion or cleaving of the extracelluar matrix. To mark, identify and study this, we employed the use of CB, a cysteine protease belonging to the family of Cathepsins which are lysosomal proteases belonging to the papain family known to get activated at lower pH. In excess of a dozen cathepsins have been recognized in numerous organisms. Of all the cathepsins, studies have shown that CB is of significant importance due to its involvement in several pathologic and cancerous processes [6]. CB, synthesized on the rough endoplasmic reticulum, is thought to enhance the activity of other proteases, mainly matrix metalloproteinase [7] resulting in matrix degradation and cell invasion.
This study seeks to examine by immunohistochemical methods using the marker CB, the said stromal changes which would point towards increased potential for invasiveness of the tumourous cells into the underlying stroma.
Materials and Methods
This was an observational study; descriptive type conducted on previously diagnosed cases of OLP were obtained from the files of the Department of Oral Pathology, Krishnadevaraya College of Dental Sciences, Bengaluru, Karnataka, India. An immunohistochemical study was performed on a total number of 70 archival blocks in the months of September 2016 and October 2016. The case details of all the patients were retrieved which consisted of 50 histologically diagnosed cases of OLP, 10 cases of normal mucosa and 10 histologically diagnosed cases of OSCC used as positive control.
Immunohistochemistry
Sections of 4 μm thickness were cut from the formalin fixed paraffin embedded blocks and mounted on poly-lysine coated glass slides. Sections were de-paraffinized and rehydrated. Endogenous peroxidase activity was blocked with 30 minutes incubation in 3% H2O2. Antigen retrieval was performed by heating the sections in a microwave at 800 W for 30 minutes with intermittent cooling after each 5 minutes period in 10mM citrate buffer (pH 6.0). Sections were thoroughly washed with Phosphate Buffered Saline (PBS) and treated with primary antibody against CB and kept for overnight incubation. Secondary antibody was applied on the sections and left in place for 30 minutes. This was then followed by application of tertiary antibody (left in place on the sections for 30 minutes, at room temperature). Sections were then washed thoroughly in PBS and the chromogen, 3,3l - Diaminobenzidine (DAB) was applied and examined microscopically for development of brown colour. Sections were then counterstained in haematoxylin for 5 minutes, dehydrated and mounted in DPX, a mixture of distyrene (polystyrene), a plasticizer (tricresyl phosphate) and Xylene. CB expression showing intensities of brown colour as a cytoplasmic staining in the epithelial cells as well as the underlying stromal cells was considered positive.
Interpretation of Staining
Presence of brown coloured end product at the site of target antigen i.e., in the epithelium was indicative of positive reactivity. The negative tissue control demonstrated absence of specific staining.
OSCC was taken as positive control, with each batch of staining. Intensity of staining was graded for statistical analysis in the ascending order, 0- absence of staining, 1 - focal staining, 2- mild staining, 3- moderate staining and 4- intense staining in all stained sections of OLP.
Statistical Analysis
With the help of SPSS software, version 20.0, Yates Chi-square test was used for statistical interpretation of the intensity of the staining results between the lesional tissues and the control groups. A p-value less than 0.05 were taken as statistically significant.
Results
All the 50 cases of OLP showed varying grades of intensities of CB immunoreactivity.
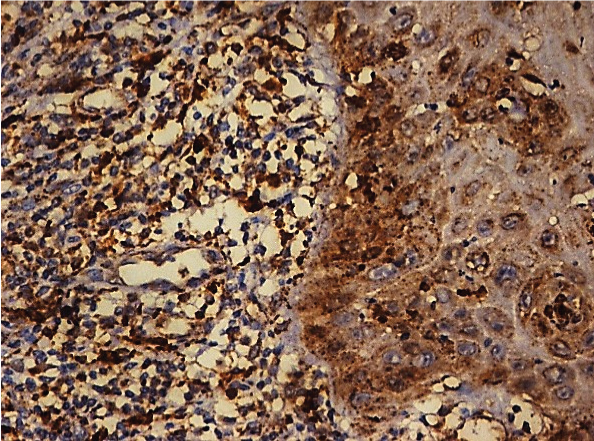
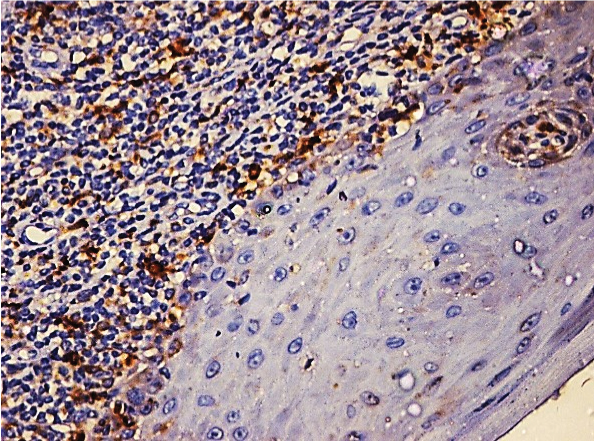
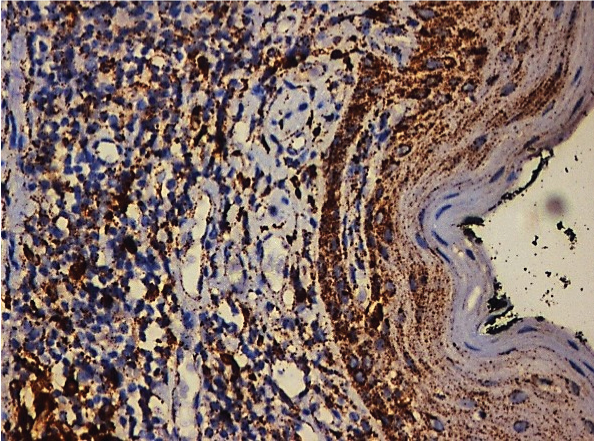
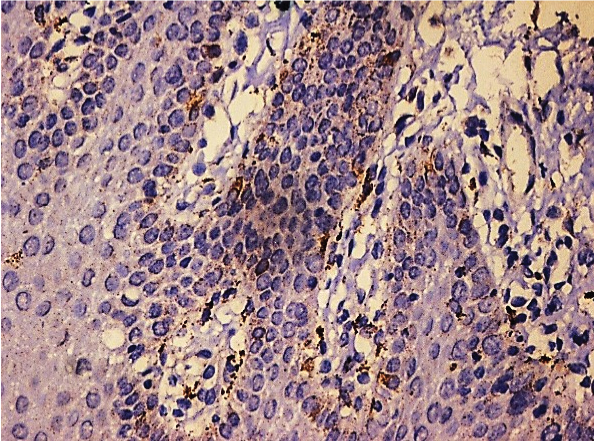
[Table/Fig-1] shows that of the 50 cases of OLP, 17 cases (34%) showed moderate staining [Table/Fig-2], 15 cases (30%) showed mild staining [Table/Fig-3], 11 cases (22%) showed intense staining [Table/Fig-4], 4 cases (8%) showed focal staining [Table/Fig-5] and 3 cases (6%) showed total absence of any stain.
Showing number of OLP, OSCC and Normal cases and their staining patterns along with their percentage. The p-value was not significant.
| Variables | Total no. of cases | Absence of stain | Focal staining | Mild staining | Moderate staining | Intense staining | Yates Chi square | p-value |
|---|
| Oral lichen planus | 50 | 3 | 4 | 15 | 17 | 11 | 14.76 | 0.06 |
| 6.00% | 8.00% | 30.00% | 34.00% | 22.00% |
| Oral squamous cell carcinoma | 10 | 0 | 2 | 3 | 2 | 3 |
| 0.00% | 20.00% | 30.00% | 20.00% | 30.00% |
| Normal tissue | 10 | 4 | 4 | 2 | 0 | 0 |
| 40.00% | 40.00% | 20.00% | 0.00% | 0.00% |
Photomicrograph showing moderate Cathepsin B expression in OLP (25X).
Photomicrograph showing mild Cathepsin B expression in OLP (25X).
Photomicrograph showing intense Cathepsin B expression in OLP (25X).
Photomicrograph showing focal Cathepsin B expression in OLP (25X).
An increase in the number of the positive cases in OLP compared to normal mucosa was seen for cytoplasmic staining for CB predominantly at the basal layers of the epithelium and in the sub-epithelial stromal cells. CB staining showed similar patterns in OSCC and the OLP group in cases of mild staining (30%). The moderate and the severe staining accounts for 56% in OLP cases and 50% in OSCC cases which is similar.
Discussion
Much controversy surrounds the malignant transformation aspect of OLP. The biggest drawback faced by researchers has been the lack of agreement on reaching a consensus on the criteria OLP is graded upon. With some studies being based purely on clinical diagnosis, some on purely histopathologic features and the others using both methods, the issue has just become more complicated, to say the least [8]. To gain better acceptance on diagnosis of OLP, Van Der Meiji E et al., suggested a modification to the WHO criteria of 1978 to diagnose OLP while stressing on the requirement of a clinic pathologic correlation in the diagnosis of OLP. He also excluded the feature of ‘epithelial dysplasia’ that seemed to have caused the maximum confusion in diagnosis of OLP. However, this seemed to have little effect as even after this conjecture, many researchers still reported considerable number of neoplastic transformation in their studies [9].
Given all these paradoxes, we wanted to hypothesize a model for malignant transformation that could be used independent of all subjective norms and more importantly is free of bias in predicting a possible malignant transformation.
Georgakopoulou EA et al., suggested that OLP exhibits a tumour-like microenvironment of the underlying stroma with an amalgamation of various coexisting factors like tissue hypoxia, presence of inflammation – releasing various cytokines and transcription factor signals, lowered vascularity, etc., [3]. He was also of the opinion that this tumour-like microenvironment is also predominated by protease activity which leads to disorganization of epithelial integrity making the epithelial cells enter into a phase of ‘cell-arrest’ and become more sensitive to cell injury. Eventually these proteases help in malignant transformation of these cells [3]. Simple as it may sound; it in fact is a complex amalgamation of various processes that are involved in reduction or complete absence in expression of intercellular and cell to matrix adhesion molecules that leads to disengagement of cells from their microenvironment. This predisposes them to invade into surrounding tissue. Dysplastic cells, mostly attributed to the existing tissue hypoxia, show increased proteolytic activity as marked by the protease Cathepsin B. These proteases help in digesting the extracelluar matrix, thereby creating a possible pathway for the cells to migrate and result in carcinomatous change [10].
The proteases known to facilitate these changes belongs to an enzyme group known to cause cleavage of the peptide bonds in various proteins. These proteases are further subclassified into five categories: matrix metalloproteinases, cysteine proteases, serine proteases, aspartic proteases and threonine proteases [11]. Cathepsins are a class of cysteine proteases and are synthesized as inactive proenzymes which are then processed to form mature and active enzymes [7]. Some cathepsins are ubiquitously expressed, such as cathepsin B, L, H, and C, whereas the newly found cathepsins K, W, and X are expressed by specific cells and tissues. The human CB gene is located at 8p22-p23.1 and the protein is widely distributed in macrophages, fibroblasts, stratified squamous epithelium, transitional epithelium, salivary glands etc., [11]. Overexpression of CB has been observed in various malignancies, including brain, lung, prostate, breast and colorectal cancer and in OSCC [5,12] With this as the background, this study was conducted to assess and analyse presence or creation of a tumour-like microenvironment in the stromal compartment of OLP which if observed, could be used to hypothesize as one of the primary mechanisms explaining the malignant shift seen sometimes in OLP cases. This could also mean that if indeed observed, the tumour-like microenvironment could singly be quoted as the main indicator in the prediction of malignant transformation even, while the subjective criteria could be kept at bay.
In our study, of the 50 cases of OLP, 17 cases (34%) showed moderate staining, 15 cases (30%) showed mild staining, 11 cases (22%) showed intense staining, 4 cases (8%) showed focal staining and 3 cases (6%) showed total absence of any stain. The positive controls that was used, in this case being OSCC, also showed quite similar patterns in staining intensity. Though it might not amount to being statistically significant, which we could explain to be due to lack of a bigger number of cases that need to be studied and corroborated with for better accuracy in results, we can certainly ascertain that the similarity goes much beyond statistical significance. In that, it points to the most important feature of invasion of cells seen in OSCC and the stromal component’s microenvironment that facilitated such an invasion. The positive reactivity of CB in the stromal component of OLP just indicates the possible mechanism that occurs in the stroma which makes it conducive for sending out disruptive signals to the epithelial cells above, which in turn would lead to their disengagement from one another and from the basement membrane, eventually leading cellular invasion and eventual malignant transformation of OLP.
Thus, in OLP lesions, epithelial cells under the influence of the underlying connective tissue stromal proteases, secreted by the inflammatory cells [13], stained by CB, have a higher tendency to transforming into cancerous cells.
Though studies appear to have not been done on proposing and agreed upon model of malignant transformation of OLP as such, we tried explaining the stromal changes as seen through CB reactivity to try and hypothesize a model that explains possible transformation.
Literature search using Medline and other search engines did not yield any other study on OLP proposing a malignant transformation model based on the interplay between an aberrant epithelial phenotype and the underlying stromal element showing a tumour-like microenvironment helping in the said malignant transformation. Therefore, our results in this category cannot be compared. With the available literature, which clearly indicates the role CB, our inference could be well applied as a possible model to explain malignant transformation.
This study might serve to be of immense therapeutic value in the sense that early and vigilant identification of the various components that make up the tumour microenvironment in patients could lead to prompt initiation of necessary treatment which will eventually lead to prevention of a possible malignant change.
Limitation
These findings need to be corroborated with more of such specific studies of CB expression in larger sample sizes of various types of OLP for more accurate results and inferences. A long term follow up is also necessary to correlate any positive malignant transformation from OLP to OSCC.
Conclusion
In this study, increased expression of CB in OLP when compared to the normal control slides and similar staining to OSCC specimen were observed. CB expression was appreciated as a cytoplasmic staining in cells of the epithelium as well as being quite clearly evident in the subepithelial stromal component. This can be explained to the already established array of mechanisms that get activated in the stromal element underneath the epithelium leading to the formation of what is known as a tumour microenvironment. Our findings using the above said parameters and diagnostic methods appear not to be reported earlier.