Cirrhosis of the liver is associated with various disturbances of the endocrine system, thought to be caused mainly by ineffective elimination of hormones by the diseased liver. It is now known that the pathogenesis of disturbed hormonal function in liver cirrhosis is rather more complex involving altered secretion and feedback mechanisms as well [1].
One such hormone in this respect is prolactin. Human prolactin is currently viewed as a hormone of pituitary origin, whose production (i.e., serum levels) is controlled by dopamine and its biological actions relate exclusively to lactation and reproductive functions [2].
Prolactin levels in patients with hepatic dysfunction have been debated in detail. Elevation of prolactin occurs mainly due to the drop in dopamine levels in the tuberoinfundibular tract [3]. Prolactin secretion is mainly regulated by tonic hypothalamic inhibition through dopamine and the stimulatory influences of hypothalamic releasing factors and circulating oestrogens. Circulating oestrogens are elevated in liver cirrhosis due to an increased peripheral aromatization of testosterone via androstenedione and to a lesser extent through a decreased elimination by the liver [4-7]. These oestrogens stimulate prolactin release by interfering with the dopamine secretion from the hypothalamus, and through a direct effect on the anterior pituitary [4].
Decompensated liver function leads to a change in the type of amino acids entering the central nervous system. Circulating concentrations of aromatic amino acids have been found to increase leading to an increase in the synthesis of false neurotransmitters such as octopamine and phenylethanolamine [8]. These false neurotransmitters may inhibit the dopamine release contributing to hyperprolactinemia [9].
With the growing incidence of cirrhosis of the liver, especially among Asian countries, prognostic criteria like the Child Pugh scoring system do not give us an idea of the probability of complications in a patient presenting with cirrhosis of the liver.
Hence, the use of a biomarker such as prolactin, whose levels give us an idea about the severity of the disease and the possibility of complications, is a very vital tool in early intervention in such cases.
The aim of this study was to assess the relation between serum prolactin levels and the severity of the disease (assessed by the Child Pugh system) in patients with cirrhosis of the liver as well as to establish that serum prolactin is an early marker for complications in a patient with cirrhosis of the liver.
Materials and Methods
This descriptive comparative study was done on 60 patients, above the age of 18 years from June 2014 to November 2015 with cirrhosis of the liver and/or its complications, with their full informed consent.
Inclusion criteria: Patients above the age of 18 years with diagnosis of liver cirrhosis and/or its complications who consented to participate in this study.
Exclusion criteria:
History of cranial surgery/ irradiation
Chest wall trauma
History of pituitary or hypothalamic disease
Chronic renal failure
Herpes zoster
Patient on medications known to elevate prolactin levels such as neuroleptics, metoclopramide, methyl dopa, reserpine, cyproterone acetate, aldosterone antagonists, morphine, cimetidine, metiamide.
Ethical considerations: Institutional Ethical Committee clearance was taken prior to the study. A written informed consent was taken from all the patients who participated in this study.
Method of data collection: The patients underwent a detailed history including past, treatment and personal history to identify possible aetiologies and a thorough clinical examination to identify the evidence of cirrhosis of the liver and the presence of its complications including portal hypertension, ascites, and hepatic encephalopathy.
Patients were subjected to the routine work up for chronic liver disease including:
Complete blood counts, urine routine, renal function tests
Liver function tests including coagulation profile
Serum prolactin at time of admission via radioimmunoassay
Ascitic fluid analysis for glucose, proteins, cytology, microbiological cultures
Upper gastrointestinal endoscopy
Ultrasound abdomen including echotexture and size of the liver, splenic enlargement and portal vein diameter
Hepatitis B Antigen (HBsAg) and anti Hepatitis C Virus (HCV) antibodies via ELISA method.
The patients were then scored based on the modified Child Pugh scoring system and divided into Classes A, B or C based on the score obtained. The complications of cirrhosis such as ascites, portal hypertension, oesophageal varices, hepatic encephalopathy, hepatorenal syndrome and spontaneous bacterial peritonitis were identified [Table/Fig-1].
Modified Child Turcotte Pugh Classification.
| Clinical andLaboratoryCriteria | Points |
|---|
| 1 | 2 | 3 |
|---|
| Encephalopathy | None | Mild to Moderate (Grade 1 or 2) | Severe (Grade 3 or 4) |
| Ascites | None | Mild to Moderate (Diuretic Responsive) | Severe (Diuretic Refractory) |
| Bilirubin (mg/dl) | <2 | 2-3 | >3 |
| Albumin (g/dl) | >3.5 | 2.8-3.5 | <2.8 |
| ProthrombinTimeSeconds prolongedInternational Normalized Ratio | <4<1.7 | 4-61.7-2.3 | >6>2.3 |
Class obtained by adding score for each parameter (total points)
Class A= 5 to 6 points (least severe)
Class B= 7 to 9 points (moderate severe liver disease)
Class C= 10 to 15 points (most severe liver disease)
Statistical Analysis
Purposive sampling was used for data analysis. Karl Pearsons’ correlation coefficient was used to compare the prolactin levels to the Child Pugh scores. Data analysis was carried out using Statistical Package for Social Science (SPSS version 10.5) package.
Results
Age and Sex Distribution: In the present study, there were 50 (83%) males and 10 (17%) females. The study included patients from 18-60 years of age with 45 (75%) patients belonging to the age group from 40-50 years, 8 (13%) patients in the age group from 30-40 years, 7 (12%) patients in the age group of 50-60 years.
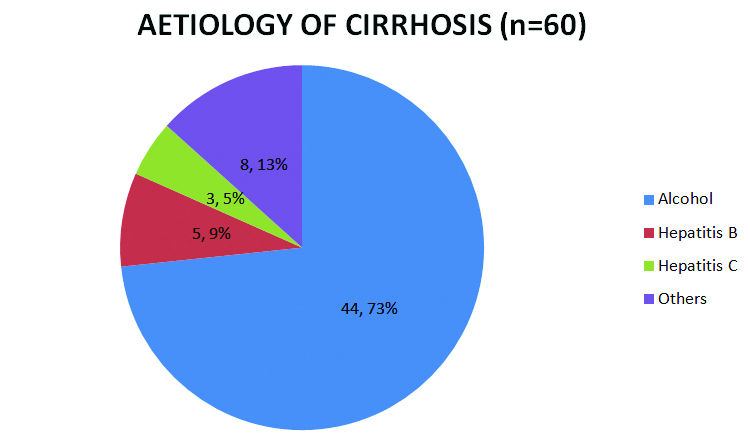
Aetiology of cirrhosis: As depicted in [Table/Fig-2], 44 (73%) of the patients were found to have alcoholic cirrhosis, 5 (9%) were hepatitis B positive and 3 (5%) were hepatitis C positive. Of the remaining eight patients, further evaluation could not be completed to establish the cause of cirrhosis.
Complications of cirrhosis noted: The complications of cirrhosis noted in our study included ascites in 53 (88.3%) patients, portal hypertension in 50 (83.3%), oesophageal varices (including portal gastropathy) in 39 (65%) with upper GI bleed in 22 (36.7%) patients, hepatic encephalopathy (grades 1-4) in 15 (25%) patients, spontaneous bacterial peritonitis in 5 (8.33%) patients and hepatorenal syndrome in 3 (5%) patients.
Child Pugh scores: Based on the modified Turcotte Child Pugh classification, 60 patients were divided into three Classes – A (score of 5-7), B (score of 7-9) and C (score 10-15). In this study, 6 (10%) patients were Class A, 24 (40%) patients were Class B and 30 (50%) belonged to Class C.
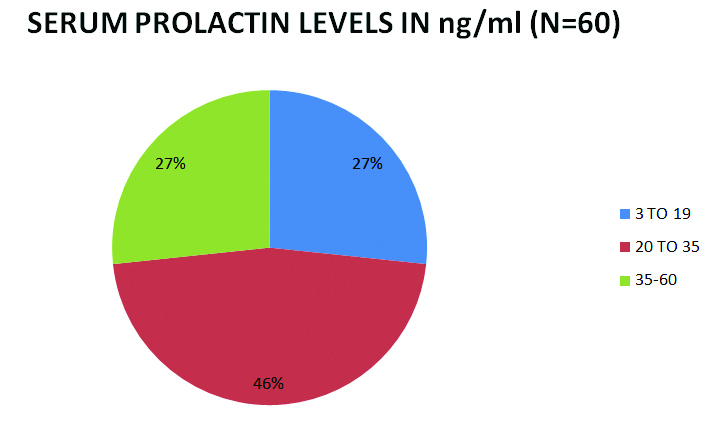
Serum prolactin levels: The normal value for serum prolactin noted in the laboratory was 3-19 ng/ml.
Of the 60 patients in this study, 44 (73.33%) of them had serum prolactin levels elevated above 19 ng/ml. The highest value of prolactin noted was 60 ng/ml [Table/Fig-3].
Comparison of serum prolactin levels and Child Pugh score: Highest levels of serum prolactin (value >35 ng/ml) were seen in patients of Class C, Child Pugh, while normal serum prolactin levels were seen in all patients but one with Class A Child Pugh.
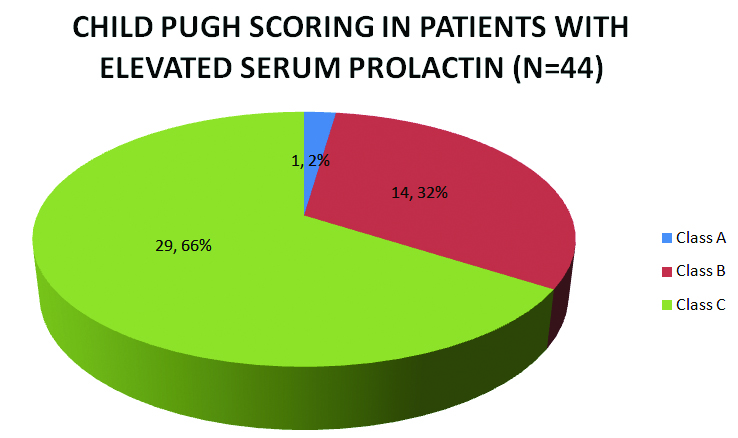
As demonstrated in [Table/Fig-4], in the 44 patients with elevated serum prolactin in our study; it was also noted that patients with a serum prolactin level above 35 ng/ml all belonged to Child Pugh Class C.
Child Pugh scores in patients with elevated prolactin.
The r-value was 0.787 which is a moderate positive correlation. The odds ratio was 19.5455, the 95% confidence interval was 2.0665 to184.8655 and the p-value was 0.0095. The odds ratio for Child Pugh Class A and elevated serum prolactin was 0.0512 with a 95% confidence interval of 0.0054 to 0.4839.
The odds ratio for Child Pugh Class B and elevated serum prolactin was 0.28 with a 95% confidence interval of 0.0848 to 0.9246.
The odds ratio for Child Pugh Class C and elevated serum prolactin was 29 with a 95% confidence interval of 3.4876 to 241.1407.
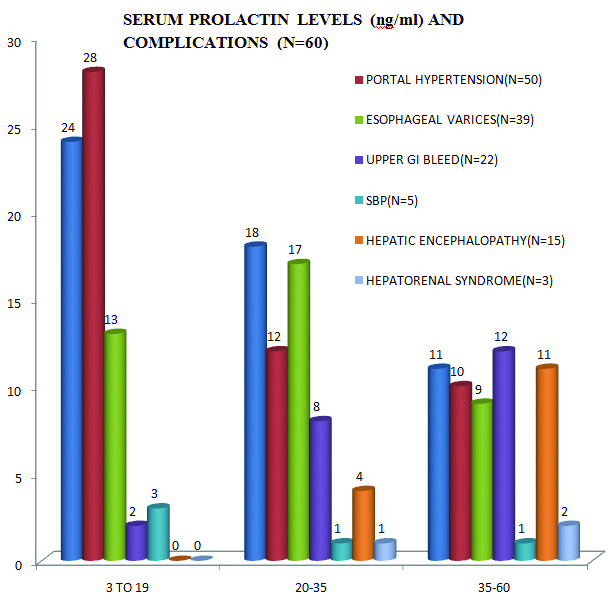
Serum prolactin levels in patients with complications of cirrhosis: In the 53 patients with ascites, 29 (54.7%) of them had elevated serum prolactin levels.
Of the 50 patients with portal hypertension, 22 (44%) had elevated serum prolactin levels.
In our study, among the 39 patients with oesophageal varices and portal gastropathy, 26 (66.7%) had elevated serum prolactin whereas 20 of the 22 patients with upper GI bleed (90.9%) were found to have raised prolactin.
Two of the 5 (40%) patients with spontaneous bacterial peritonitis had elevated serum prolactin.
All patients with hepatic encephalopathy (15) and hepatorenal syndrome (3), i.e., 100%, had elevated serum prolactin including the highest reading noted in our study of 60 ng/ml [Table/Fig-5].
Serum prolactin levels with relation to the complications of cirrhosis.
Mortality: In our study, deaths were recorded in 17 of the 60 patients (28.33%) and it was observed that all 17 patients had elevated serum prolactin level.
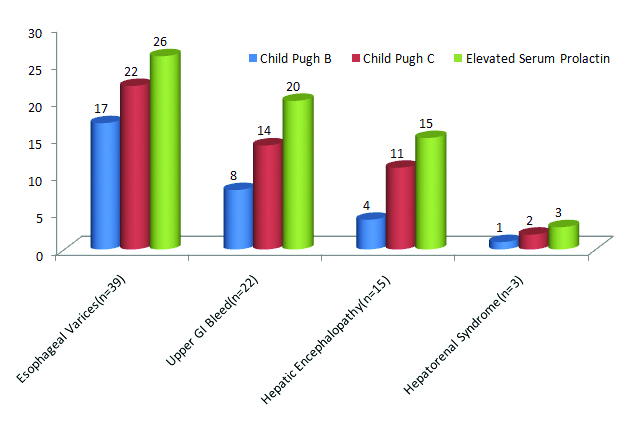
Serum prolactin values compared to Child Pugh class in complications of cirrhosis: For each complication of cirrhosis, the graph compares the incidence of elevated serum prolactin and Child Pugh classes B and C. As depicted, in all the major complications of cirrhosis, there is elevated serum prolactin as well as a higher Child Pugh Class (B or C) [Table/Fig-6].
Comparison of elevated serum prolactin and Child Pugh Class B and C in complications.
Discussion
The study was done on 60 patients with cirrhosis of the liver and its complications in order to assess the efficacy of serum prolactin in comparison to the Child Pugh scoring as well as to study its ability to detect the complications early in their course.
Our study on 60 patients had a male to female ratio of 5:1 with the 75% of the patients in the age group of 40-50 years of age. The aetiology of cirrhosis most commonly found in our study was attributed to alcoholic liver disease (73%) followed by hepatitis B infection (9%). A similar study on 70 patients by Velissaris D et al., showed a median (range) age in years of 56 (34-68) and male: female ratio of 2:1. Of the total 60 patients with liver disease, 19 were found to be positive for HBsAg (13 with viral hepatitis and six with cirrhosis liver) [3].
The Child Pugh class distribution in our study showed 10% belonging to Class A, 40% to Class B and 50% to Class C when compared to the study by Velissaris D et al., where 34.3% patients with liver cirrhosis belonged to category A of Child Pugh classification, 22.9% were B and the remaining 42.9% were C type [3].
The serum prolactin levels in our study were elevated in 73.33% of the patients and higher prolactin levels were noted in patients with higher Child Pugh classes (B and C). This was similar to the findings in a study done by Arafa M et al., who found that prolactin levels increased as the Child Pugh class increased from A to C [10].
Our study revealed that higher serum prolactin levels were found in patients with the complications of cirrhosis of the liver such as hepatic encephalopathy, hepatorenal syndrome, coagulopathy, oesophageal varices with upper GI bleed, spontaneous bacterial peritonitis. This was similar to the findings in a study by Koller T et al., who found that higher prolactin scores were seen in patients with higher ascites and encephalopathy stage as well as higher INR [11].
It was also observed that higher prolactin levels were seen in patients with Child Pugh Class B and C (highest, 60 ng/ml in a patient with Class C) which was similar to the study by Zeitz B et al., revealed prolactin to be highest in patients with Child Pugh Class C [12].
All the patients with hepatic encephalopathy and hepatorenal syndrome and 90% of the patients with an upper GI bleed had markedly elevated serum prolactin levels and this was similar to the findings by Arafa M et al., who found that patients with hepatic encephalopathy had high prolactin levels which increased with the stage of encephalopathy [10].
Limitation
This was a study done with a small sample size. There was no correlation established with the aetiology of the liver cirrhosis. The extent of prolactin elevation was not compared to the incidence of complications.
Conclusion
The study shows that serum prolactin levels in patients with cirrhosis of the liver can serve as a marker for the severity of the disease as it closely correlates with the Child Pugh scoring system.
It also demonstrates that serum prolactin levels are significantly higher in patients with complications such as hepatic encephalopathy and higher the levels, greater the severity.
Hence, we conclude that serum prolactin levels can be used as a useful prognostic marker in patients with cirrhosis of the liver as well as an early indicator of its complications.