Sarcomatoid Carcinoma of Renal Pelvis with Abundant Heterologous Osteosarcomatous Element: A Case Report
Mona Lisa1, Guddi Rani Singh2, Richa Madhawi3, Bipin Kumar4, Zeenat Sarmadi Imam5
1 Senior Resident, Department of Pathology, Indira Gandhi Institute of Medical Science, Patna, Bihar, India.
2 Senior Resident, Department of Pathology, Indira Gandhi Institute of Medical Science, Patna, Bihar, India.
3 Assistant Professor, Department of Radiotherapy, Regional Cancer Center, Indira Gandhi Institute of Medical Science, Patna, Bihar, India.
4 Professor and Head, Department of Pathology, Indira Gandhi Institute of Medical Science, Patna, Bihar, India.
5 Senior Resident, Department of Pathology, Indira Gandhi Institute of Medical Science, Patna, Bihar, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mona Lisa, 401 D Ashirwad Enclave, Anandpuri, Patna-800001, Bihar, India.
E-mail: monaloud@gmail.com
A 47-year-old male presented with haematuria and flank pain for two weeks. Ultrasonography and renal scan revealed a poorly functioning left kidney with multiple calculi. Simple nephrectomy was performed and the specimen revealed a mass in his renal pelvis which showed both carcinomatous and sarcomatous components on microscopy. The sarcomatous component consisted of diffuse pleomorphic osteoblasts with intervening lacy osteoid, giving an osteosarcoma-like appearance. These areas of tumour were strongly positive for vimentin and osteopontin. The carcinomatous component was transitional cell carcinoma. Patchy areas of squamous cell carcinoma which were positive for pancytokeratin on immunostaining were also seen. Few weeks later, the patient presented with metastatic lesions in the sacrum. After nephrectomy, the patient underwent palliative radiotherapy of the spine followed by sunitinib therapy. A month later, there was recurrence at the site of surgery. The patient succumbed to his illness within five months of diagnosis. This report describes an extremely rare case of carcinoma, renal pelvis with predominantly osteosarcomatous areas.
Kidney, Necrosis, Osteosarcoma renal pelvis
Case Report
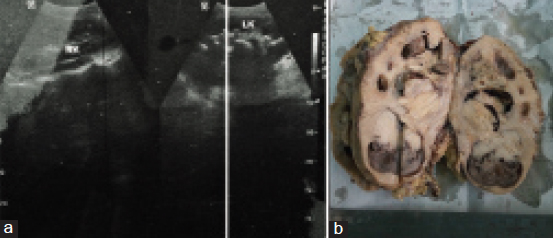
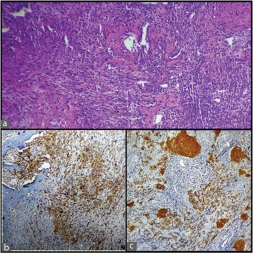
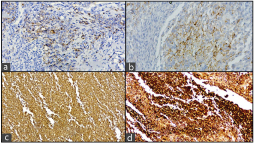
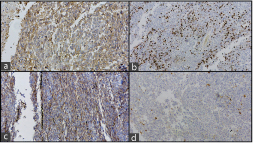
A 47-year-old male, tobacco chewer, hailing from Gaya, Bihar, India, presented with a 15-days history of left flank pain and haematuria. His blood urea nitrogen and creatinine were elevated, and hemoglobin was low. Rest of blood chemistry was within normal limits. Ultrasonography showed left hydronephrotic kidney with multiple calculi [Table/Fig-1a]. Nephrectomy was done considering a poorly functioning left kidney on renal scan. The resected kidney was enlarged and the cut surface revealed a tumour measuring 7 cm × 5 cm × 4.5 cm in the pelvis pushing the cortex along with multiple calculi 2-3 cm in diameter. The tumour was solid grayish brown on cut, with areas of hemorrhage and necrosis [Table/Fig-1b]. Microscopically, the tumour showed mostly osteosarcoma-like areas with osteoblastic tumour cells in a background of lacy osteoid and giant cells. Some areas showed high-grade transitional cell carcinoma with pleomorphic tumour cells in sheets along with focal islands of malignant squamous cells. The tumour was focally invading the renal capsule and resected margin of ureter was free of tumour. Lymphovascular invasion was not seen in the sections. The stage of the tumour was pT4N0Mx. Immunohistochemical study revealed that the sarcomatous areas were diffusely positive for vimentin and osteopontin, and the areas with malignant urothelial cells/squamous cells were positive for pancytokeratin (AE1/AE3) and Epithelial Membrane Antigen (EMA). The Ki67 proliferation index was 40. The sections were also diffusely positive for CD99 and patchy positive for smooth muscle actin while they were negative for S-100 [Table/Fig-2,3 and 4].
a) Ultrasonography showed left hydronephrotic kidney with multiple calculi; b) Gross specimen.
Photomicrographs showing: a) Hematoxylin and eosin stained section of tumour (X10); and b,c) Pancytokeratin positive urothelial cells and squamous cells on immunohistochemistry (X4).
Photomicrographs showing focal positivity for: a) Pancytokeratin (X40); b) Epithelial membrane antigen (X40); and c,d) Diffuse positivity for vimentin and osteopontin (X4).
Photomicrographs showing a) CD99 positive (X40); b) Ki 67 index 40%; c) Smooth muscle actin positive (X4); d) S100 negative stain (X4).
By the time, the histopathology report was dispatched the patient had developed metastasis in the sacrum. He underwent palliative radiotherapy of the spine followed by sunitinib. One month later, he developed recurrence at the site of surgery. The patient succumbed to his illness in <5 months.
Discussion
Transitional cell carcinoma is the most common type of malignancy of the urinary tract followed by squamous cell carcinoma and adenocarcinoma. Sarcomatoid carcinoma of the renal pelvis is a very rare histological type with <15 cases reported till date [1]. Most of the urothelial carcinomas of the renal pelvis are of higher grade and present at advanced stages compared to the urothelial carcinomas of the urinary bladder [2].
Sarcomatoid carcinoma is a high-grade malignant neoplasm with a biphasic microscopic appearance. According to the World Health Organization Classification of Tumours 2004, the term “sarcomatoid carcinoma” should be used for all biphasic malignant neoplasms that show morphological and/or immunohistochemical evidence of epithelial and mesenchymal differentiation [3]. The epithelial elements express cytokeratins and sarcomatous elements react with vimentin or other mesenchymal specific markers [4,5]. In our case, the tumour showed both pankeratin (AE1/AE3) and vimentin positivity. In most cases, the sarcomatoid carcinoma consists of poorly formed fascicles of undifferentiated spindle cells [4]; however, in our case, there was exuberant bone formation, with atypical osteoclast giant cells and abundant osteoid which was strongly positive for osteopontin. In between, there were areas of malignant transitional cells and squamous cells which showed pancytokeratin positivity.
In a study done by Lopez-Beltran A et al., the image DNA ploidy of all the cases of sarcomatoid carcinoma of the renal pelvis showed an aneuploid pattern distinguishing them from the pseudosarcomatous tumours [5]. In another study, Gronau S et al., described that the occurrence of overlapping chromosomal aberrations strongly argues for a monoclonal origin of this tumour with a common ancestor and a divergent course of tumour progression in both components [6].
The main differential diagnosis in our case was primary osteosarcoma of the kidney, however, malignant urothelial cells and squamous cells, positive for pancytokeratin, did not favor it.
Sarcomatoid carcinoma of the renal pelvis commonly presents with haematuria, dysuria, nocturia, acute retention of urine, and lower abdominal pain [7]. Our patient also presented with flank pain and haematuria.
It has been established that patients with sarcomatoid variant of urothelial carcinoma have worse disease-specific and overall survival [8] which was around five months for our patient.
Conclusion
To sum up, it was a case of sarcomatoid carcinoma arising in the renal pelvis with predominantly osteosarcomatous differentiation and focal areas of papillary urothelial and squamous cell carcinoma. It is a very rare tumour with worse prognosis.
[1]. Chen S, Chen G, Xia D, Li J, Wang S, Shen B, Sarcomatoid carcinoma of the renal pelvis: Experience of multiple cases over a ten-year periodOncol Lett 2013 6(2):513-16. [Google Scholar]
[2]. Perez-Montiel D, Wakely PE, Hes O, Michal M, Suster S, High-grade urothelial carcinoma of the renal pelvis: Clinicopathologic study of 108 cases with emphasis on unusual morphologic variantsMod Pathol 2006 19(4):494-503. [Google Scholar]
[3]. John NE, Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs 2008 1st edLyonIARC Press [Google Scholar]
[4]. Sanfrancesco J, McKenney JK, Leivo MZ, Gupta S, Elson P, Hansel DE, Sarcomatoid urothelial carcinoma of the bladder: Analysis of 28 cases with emphasis on clinicopathologic features and markers of epithelial-to-mesenchymal transitionArch Pathol Lab Med 2016 140(6):543-51. [Google Scholar]
[5]. Lopez-Beltran A, Escudero AL, Cavazzana AO, Spagnoli LG, Vicioso-Recio L, Sarcomatoid transitional cell carcinoma of the renal pelvis. A report of five cases with clinical, pathological, immunohistochemical and DNA ploidy analysisPathol Res Pract 1996 192(12):1218-24. [Google Scholar]
[6]. Gronau S, Menz CK, Melzner I, Hautmann R, Möller P, Barth TF, Immunohistomorphologic and molecular cytogenetic analysis of a carcinosarcoma of the urinary bladderVirchows Arch 2002 440(4):436-40. [Google Scholar]
[7]. Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, Carcinosarcoma and sarcomatoid carcinoma of the bladder: Clinicopathological study of 41 cases.J Urol 1998 159(5):1497-503. [Google Scholar]
[8]. Black PC, Brown GA, Dinney CP, The impact of variant histology on the outcome of bladder cancer treated with curative intentUrol Oncol 2009 27(1):3-7. [Google Scholar]