Epithelial dysplasia may occur because of cytological and structural alteration during epithelial cell renewal and maturation [1,2]. Carcinogenesis is a genetic process that leads to change the cellular morphology and behavior [3]. In oral cancer, a similar process with accumulation of genetic alterations is shown with different manifestation such as hyperplasia to dysplasia or carcinoma in situ to malignancy [3]. Several studies have been conducted to discover new treatments such as the use of phytotherapy in the treatment of neoplasms [4,5]. The purpose of using these substances is inhibiting, delaying, or even eliminating carcinogenesis process [5].
Propolis contains flavonoids, waxes, polyphenols, phenolic acids, phenolic aldehydes, ketones, and herbal pollens [6]. Propolis has many biological effects such as anti-inflammatory, antifungal, antiviral, antioxidants [7-12], and wound healing [7,13] and has the ability to stop the proliferation of cancer cells [14] and controlling tumor growth and metastases [15].
One study showed that propolis has an inhibitory effect on the growth of cancer cells from human breasts [16], and there are many studies to show the similar effects for chemical compositions of propolis [2,7,11,15].
Based on the biologic effects of propolis on the growth of cancer cells and previous studies conducted in the field, the purpose of this study was to evaluate the effect of Iranian propolis on dysplastic changes of oral mucosal epithelium in an animal model.
Materials and Methods
This was an interventional (experimental or animal) study, and sampling was done based on random selection of 28 male Wistar rats (7-11 weeks, 160±20 g). This project was approved by Research and Ethics committee of Babol University of Medical Sciences. (Ethical number: 9133625). After one week of acclimatization, the rats were placed in metal laboratory cages at standard conditions (temperature: 22±2°C, dark/light cycles; 12/12 h). They had access to food (including cabbage, carrots, and a material called plate 21) and water ad-libitum [17].
In this study, the chemical carcinogen 7,12-Dimethylbenz[a] anthracene (DMBA) (Sigma-Aldrich, USA) and fresh propolis (produced in the Mazandaran province, Mazandaran, Iran) were used. For making propolis as an injectable solution, Tween 80, water, and ethanol were used.
Rats were divided into four groups. Group 1: As control group which received 0.5% DMBA and the combination of water, ethanol, and Tween 80; Group 2 received 0.5% DMBA with 100 mg/kg propolis; Group 3: 0.5% DMBA with 200 mg/kg propolis; Group 4: 0.5% DMBA with 400 mg/kg propolis [Table/Fig-1].
Distribution of the control and experimental groups.
| Groups | Number of rats | 0.5% DMBA (brush) | Propolis (IP) |
|---|
| Group 1 | 7 | ✓ | - |
| Group 2 | 7 | ✓ | 100 mg/kg |
| Group 3 | 7 | ✓ | 200 mg/kg |
| Group 4 | 7 | ✓ | 400 mg/kg |
Abbreviations: DMBA: 7,12-dimethylbenz[a]anthracene, IP: Intraperitoneal.
One gram of DMBA solved in benzene up to 0.5% of concentration [18,19]. In all four groups of rats, tongue pulled out by forceps and made them immobile. Then, the solution of DMBA was applied topically on the middle third of the dorsal surface of the tongue of rats, twice on every alternate day for 20 weeks.
Providing of Propolis Extract
To obtain a homogenous propolis solution, solid propolis, Tween 80, water and ethanol were used. For achieving a uniform mixture, propolis was dispersed in 5 ml of ethanol and 10 ml of Tween 80, after all 70°C distilled water was added. On the hot plate at a temperature of 50-60°C, the mixture was stirred for six hours [20].
Then, 10 min before each injection, the mixture was stirred at room temperature, and the rats were injected intraperitoneal (IP) three times a week for 20 weeks (in the days after use of DMBA).
After 20 weeks, rats were sacrificed for postmortem removal of the painted area. Tissue specimens were fixed in formalin 10% (pH 7.4, 10%) for 24 hours, and then paraffined blocks were prepared. Histological sections were 5 µm thick, which were stained by hematoxylin-eosin and evaluated by an oral pathologist using a light microscope.
Some manifestations such as lack of polarity of basal layer, abnormal variation in nucleus size, nuclear pleomorphism, abnormal variation in cell size, cell pleomorphism, increased nucleus/cytoplasm ratio, increased size of the nucleus, abnormal mitotic figures, increased number, and size of nucleoli were considered.
Dysplastic changes were categorized into: Mild dysplasia, if the changes were confined to the lower third of the epithelium (basal and parabasal layers); moderate dysplasia, when the changes were seen in the middle third of the epithelium (middle layers of the squamous layer); severe dysplasia, when architectural and cytologic changes were found beyond the middle third of the epithelium [3].
Three images at 10X magnification were prepared from each slide using the Olympus DP12 camera mounted on Olympus BX41 microscope (Tokyo, Japan). The thickness of keratinous layer and epithelium in three points for each sample was obtained using Analysis SLS Starter software, and the average was calculated.
Statistical Analysis
To compare the severity of dysplasia between the groups, Kruskal–Wallis test and comparison of the epithelial thickness and keratinous layer ANOVA post-hoc test was performed, and p<0.05 was considered significant.
Results
Twenty-eight male rats were divided into four equal groups. During the 20 weeks of the study, all the rats were alive except one of them that received propolis 200 mg/kg. After 20 weeks, all of rats were euthanized, and histopathological studies of the specimens from their tongues were performed.
Dysplasia
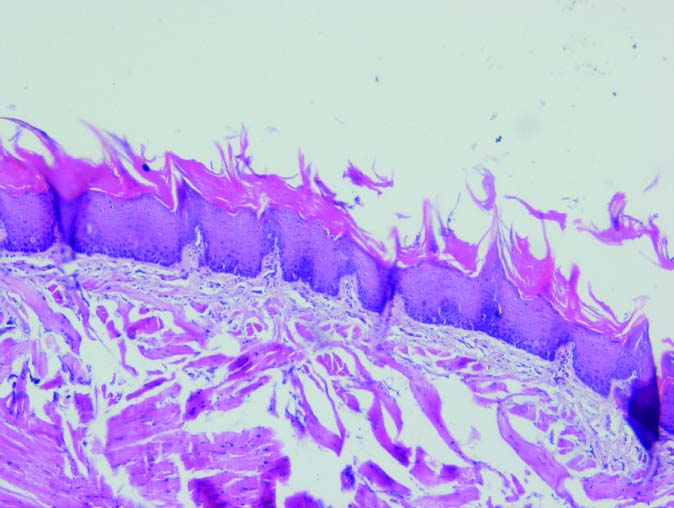
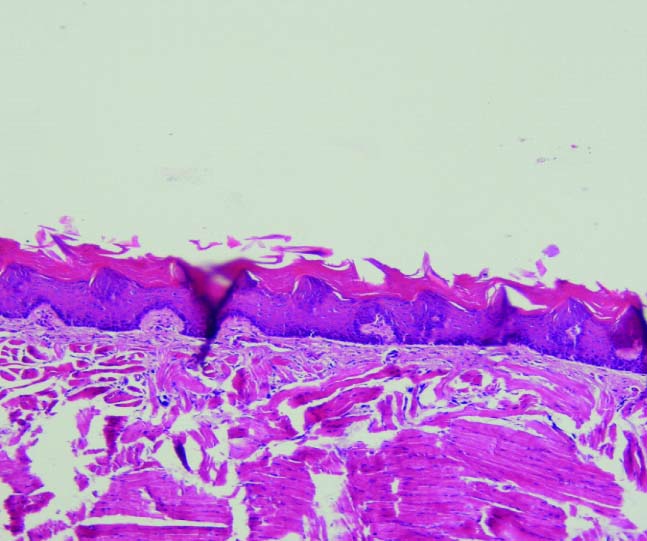
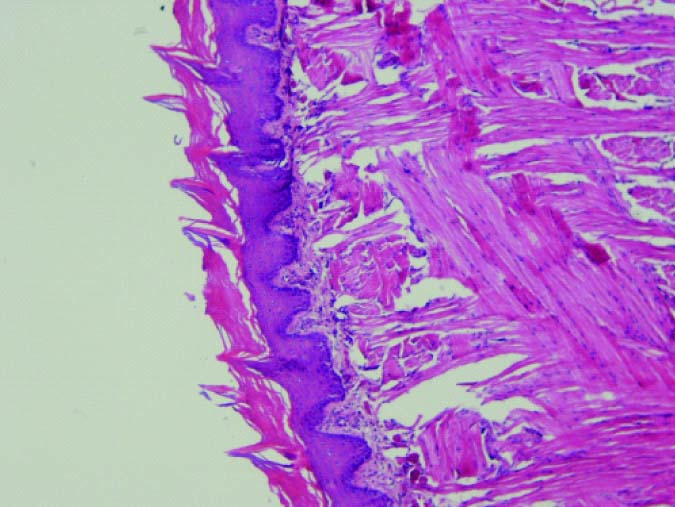
Histopathologic evaluation showed that in the group which received 400 mg/kg propolis; two rats showed mild dysplasia and five rats were normal. The group received 200 mg/kg propolis, five cases showed mild dysplasia; one rat was normal and one rat died. The group that received propolis 100 mg/kg, five cases had moderate dysplasia and two cases had mild dysplasia. In the control group, six rats showed moderate dysplasia and one showed mild dysplasia [Table/Fig-2,3,4 and 5].
Moderate dysplasia in Group 1 (10X).
Mild dysplasia in Group 2 (10X).
Mild dysplasia in Group 3 (10X).
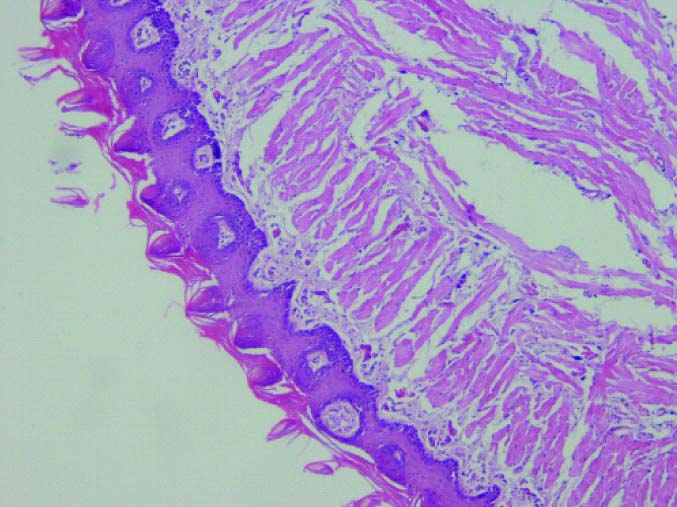
In this study, findings suggest that by increasing dose of propolis; the degree of dysplasia decreases (dose of propolis inversely related to the severity of dysplasia). As in the control group, dysplastic cells were reached to the middle third of the epithelium while in Groups 2 and 3, which were received propolis 100 mg/kg and 200 mg/kg; atypical cells were seen in the basal region only. In Group 4, which received 400 mg/kg of propolis, epithelial dysplasia was not observed in most cases. The difference was a significant between the control and each groups in terms of severity of dysplasia (Group 2 [100 mg/kg propolis] [p=0.017], Group 3 [200 mg/kg propolis] p=0.02, and Group 4 [400 mg/kg propolis] [p=0.002].
In this study, thickness of epithelium in control groups showed a significant difference with groups which received propolis [Table/Fig-6] so that the minimum thickness of the epithelium was observed in the control group. Thickness of keratinous layer between control and case groups did not show any significant differences.
Mean (SD) of epithelium thickness and keratinous layer thickness of study groups.
| Treatment groups | Mean (SD) |
|---|
| Epithelium thickness | Keratinous layer thickness |
|---|
| Control (DMBA) | 58.6 (15.3) | 42.1 (19.6) |
| Propolis 100 mg/kg | 101.9 (35.7) | 64.8 (23.9) |
| Propolis 200 mg/kg | 103.6 (13.5) | 58.9 (17.0) |
| Propolis 400 mg/kg | 124.5 (35.5) | 71.4 (20.9) |
| p-value | 0.002 | 0.078 |
ANOVA post-hoc test. Abbreviations: DMBA: 7,12-dimethylbenz[a]anthracene, SD: Standard deviation.
Discussion
This study evaluated the effect of Iranian propolis on dysplastic changes of the epithelium after administration of carcinogens (DMBA) on the tongue of rats. In this study, propolis could reduce the severity of dysplasia in the mucosa in an animal model. Hence, this effect was dose dependent.
The results of this study showed propolis has antitumor effects, as the severity of dysplasia restricted in the lower third of the epithelium in group which received propolis. Whereas, in control group, dysplastic cells were also found in the middle third of the epithelium too that can be caused by antitumor properties of propolis, which was mentioned in Cavalcante, Brazil study [19]. It was performed on an animal model with applying propolis and showed its antitumor effect. This effect is attributed to the presence of flavonoids as one of its ingredients [19].
In Cavalcante’s study, that propolis was administered by gavage. The results were similar with this study. Cavalcante’s study showed the severity of dysplasia in groups which received propolis were significantly lower than the control group, and Caffeic Acid Phenethyl Ester (CAPE) has been proposed as the main ingredient of propolis that can inhibit protein and RNA synthesis [19]. In the other in vitro and in vivo studies (outside of oral mucosa) propolis was injected IP, and CAPE is known as a major antitumor agent with the ability to inhibit DNA synthesis on cultured tumor cells and induce apoptosis in them [15,21]. Furthermore, its cytotoxic effects on pancreatic cancer and human colorectal cells with inducing of apoptosis were found. In investigation of cancerous cell, morphology has been shown that apoptosis and activity of the complement cascade in CAPE group compared with the control group was two times higher [21].
Propolis differs in composition due to differences in local vegetations, pharmacological activity of propolis is highly variable depending on its geographic origin [17,22]. In our study, reduced severity of dysplasia in cases, groups might be related to the presence of these active ingredients in propolis. In addition, increase in the concentration of propolis, lead to decreasing grade of dysplasia which suggests dose-dependent effects of propolis. However, further studies are needed.
The minimum thickness of the epithelium was seen in the control group; also, epithelial thickness was increased with increasing dose of propolis. It seems that carcinogenic substances can create atrophic epithelium, but propolis can provide normal keratinized epithelium and can decrease the carcinogenic effects on the epithelium. It is recommended that the effect of propolis on the thickness of oral epithelia with the exposure of the carcinogenic substances to be considered in future studies.
Alizadeh AM et al. study in 2015 examined the chemoprotective role of Iranian propolis on the growth of cancer cells in the gastrointestinal tract. Cancer propolis in the study was assessed by the ultra performance of liquid chromatography-mass spectrometry analysis. Based on results, Iranian propolis contains high amounts of flavonoids including caffeic acid and pinobanksins. The percentage of CAPE in Iranian propolis was found to be significantly higher. It was also mentioned that CAPE at low doses as well prevented cellular mistakes in healthy cells and induces apoptosis. CAPE possesses potent inhibitory effect on cell proliferation, shows powerful antioxidant activity. Overall, it has a cytotoxic effect and blocks the invasive metastasis noted in tumors [22].
Another study in Brazil in 2015 investigated that the effect of different concentration of hydroalcoholic extract of Brazilian red propolis (HERP) exerted on DMBA-induced oral squamous cell carcinoma and concluded that propolis in concentrations of 50 and 100 can have an inhibitory effect on 40% of Oral Squamous Cell Carcinomas (OSCCs). Finally, they suggested that HERP exerts chemopreventive activity on the progression of DMBA-induced epithelial dysplasia to OSCC in an experimental model of labial carcinogenesis [23]. The study limitations included difficulty in working with animals when injected propolis and possible death rats during this study.
Conclusion
On the basis of this study and other studies about the antitumoral effects of propolis, significant connection between increasing concentrations of propolis and antitumoral effects of this material on oral mucosa seems probable and recommends in future studies the mechanism of this effect should be examined clearly.
In this study, the protective effect of propolis on carcinogenesis of oral epithelium was shown and proved. The better understanding of this mechanism requires further studies.
Abbreviations: DMBA: 7,12-dimethylbenz[a]anthracene, IP: Intraperitoneal.