Leukoencephalopathy is a progressive white matter disease primarily caused due to myelin damage by a variety of factors. Chemotherapy for oncological treatment is an uncommon but important cause of potentially reversible leukoencephalopathy. In current radiological setting, conventional and diffusion weighted MRI play a significant role in early and accurate detection of this entity. We are hereby presenting MRI evaluation of two cases of methotrexate and 5-fluorouracil induced toxic leukoencephalopathy.
Diffusion weighted imaging, Methotrexate, Neurotoxicity, Toxic encephalopathy
Case Report
Case I
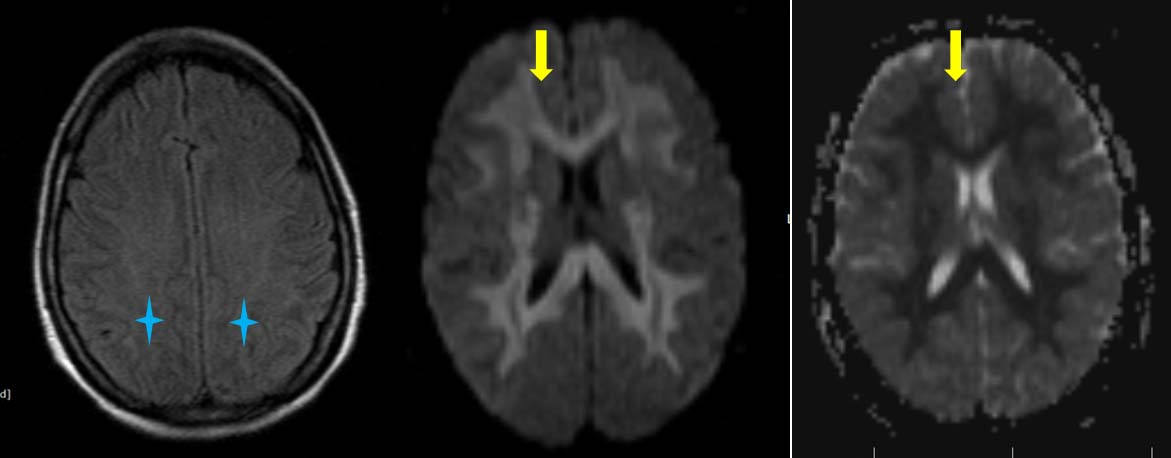
A 35-year-old female patient, known case of Stage IIIB carcinoma colon, presented to emergency department with altered sensorium, four days after administration of chemotherapy 5-Fluorouracil (5-FU). Blood investigations including blood glucose, serum bilirubin, alkaline phosphatase, serum creatinine were in normal limits. Magnetic Resonance Imaging (MRI) brain was done to rule out acute ischemic event. Study revealed mild T2 and FLAIR hyperintensity of white matter of bilateral cerebral hemisphere in fronto-parieto-temporo-occipital region, bilateral internal capsule, corpus callosum, bilateral superior cerebral peduncle and bilateral middle cerebellar peduncle. These areas exhibited restriction on Diffusion-Weighted (DW) and Apparent Diffusion Cofficient (ADC) images as shown in [Table/Fig-1]. No evident enhancement was seen on contrast enhanced images. In lieu of the clinical history and corresponding imaging findings, provisional diagnosis of chemotherapy induced toxic leukoencephalopathy was kept. Treatment with 5-FU was thereafter stopped and patient was further treated with oxaliplatin. Patient recovered after 15 days with complete reversal of MRI findings on repeat MRI scan.
a) Axial FLAIR sequence showing subtle hyperintensity of white matter of bilateral cerebral hemispheres (blue star); b,c) DWI and ADC images showing diffusion restriction (yellow arrows) in FLAIR hyperintenseareas. ADC values (10-3 mm2/s) were found to be 0.320 to 0.450 in affected white matter and 0.881 in unaffected white matter.
Case II
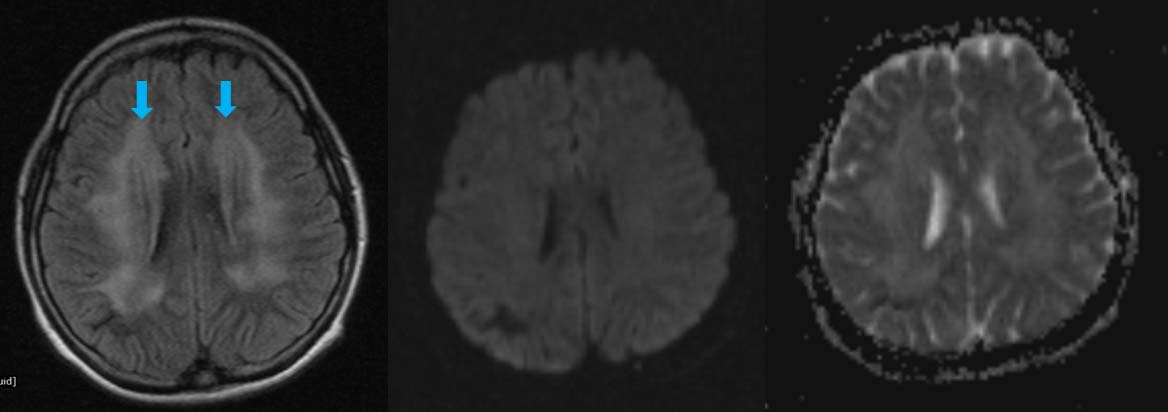
An 18-year-old female patient, who was a known case of Acute Lymphoid Leukaemia (ALL) on intrathecal chemotherapy {methotrexate-(MTX)}; presented with convulsions since six months. MRI done in early phase of treatment revealed no significant findings. Subsequent MRI, six months later revealed confluent, symmetrical, altered signal intensity areas in bilateral centrum semi-ovale and bilateral corona radiata, peri-ventricular deep white matter adjacent to frontal horn and occipital horns of both the lateral ventricles with sparing of bilateral subcortical “U” fibers; which appeared hyperintense on FLAIR and T2W images, isointense to hypointense on T1W images. Lesions did not show restriction on DW and ADC images as shown in [Table/Fig-2]. There was no obvious blooming on gradient images or contrast enhancement noted on post-contrast images. Possible diagnosis of chemotherapy induced toxic leukoencephalopathy was given. Methotrexate was stopped after the imaging diagnosis and supportive treatment was given with anti-oxidants. Though the patient clinically improved, there was no follow up imaging.
a) Axial FLAIR sequence showing hyperintensity of white matter of bilateral centrum semi-ovale and bilateral corona radiate (blue arrows); b,c) DWI and ADC sequence showing no areas of restriction. ADC values (10-3 mm2/s) were found to be 1.032 in affected white matter and 1.017 in unaffected white matter.
Discussion
Leukoencephalopathy is a condition characterized by progressive structural damage to the cerebral white matter resulting primarily from damage of myelin. Amongst a spectrum of endogenous and exogenous causes such as metabolic, infectious, radiation, toxic and environmental, toxic leukoencephalopathy caused by chemotherapeutic agents is worth discussion as it is a potentially reversible situation if the offending agent is withdrawn before onset of irreversible damage [1].
In an oncological set up, a strong clinical suspicion should be made for acute toxic leukoencephalopathy in a patient who presents with recent onset of neurologic symptoms and has known exposure to a drug which is a potential agent for causing injury to the white matter. The chemotherapeutic agents known to cause such neurotoxicity include methotrexate, 5-FU, vincristine, cyclosporine, ifosfamide, fludarabine and cisplatin. Methotrexate and 5-FU are the most commonly reported offenders [1-3].
Methotrexate, an important part of the treatment protocol of childhood leukaemias, is a cell cycle-specific agent that inhibits dihydrofolatereductase, preventing the conversion of folic acid to tetrahydrofolic acid and thereby inhibiting cell replication. Both high-dose intravenous MTX and intrathecal MTX are associated with severe white matter damage. MTX also causes a relative excess of homocysteine, a byproduct of the folate deficiency, which is thought to induce small-vessel vasculopathy [4]. Incidence of acute neurotoxicity from MTX is 3%-10% which is aggravated by presence of various factors such as dose, route of administration (intrathecal more dangerous), young age and associated cranial irradiation [4].
5-FU is a fluorine-substituted analogue of pyrimidine uracil which is used in treatment of solid cancers such colon, stomach, ovary and head and neck. The main action of this agent is to block DNA synthesis by reducing the formation of thymidine monophosphate and incorporation into RNA [5]. 5-FU readily penetrates the blood-brain barrier. It has a reported neurotoxicity rate of around 5% which is more in females and patients with malnutrition or liver dysfunction [5].
The clinical presentation can vary from headache, dizziness and depression when the toxic agent is taken in low doses to more severe symptoms like confusion, seizures; acute, subacute and chronic ischemic attacks, posterior reversible encephalopathy syndrome, cerebellar dysfunction and myopathy [6].
On CT imaging, bilateral relatively symmetrical hypodense areas may be seen in the involved white matter. On conventional MRI, toxic leukoencephalopathy commonly appears as diffuse T2 and FLAIR hyperintense signal in deep periventricular white matter and corpus callosum with sparing of basal ganglia, thalamus and subcortical U fibers. Post gadolinium images may or may not show any abnormal contrast enhancement [7].
Nowadays, the onus of radiological diagnosis lies with Diffusion Weighted Imaging (DWI) which is quick in detection of findings before their appearance on FLAIR images. On DWI focal or diffuse areas of reversible restricted diffusion with low ADC can be observed in acute cases, which may show improvement over the period of time if the drug is stopped. However, the corresponding changes in conventional MRI such as T2 and FLAIR hyperintensities may show a larger transitional period for improvement [8].
DWI is based on motion of water molecules. In acute ischemia, diffusion restriction is seen; the cells which have been irreparably damaged by the ischemia and hence DWI is a marker of cytotoxic oedema. However, this is not the only situation in acute toxic leukoencephalopathy where the pathological basis of disease lies in myelin destruction from various causes such as; small vessel vasculopathy, cytotoxicity from endothelial damage or direct toxic demyelination; which results insegmental splitting, vacuolization and release of multiple vacuoles. The accumulation of these vacuoles within the myelin restricts water movement thereby resulting in diffusion restriction. Though, DWI is an early and accurate way of diagnosing a toxic white matter insult, it does not necessarily indicate towards a poor prognosis and the tissue may still be salvageable [4,5,9].
Imaging differential diagnoses for bilateral white matter lesions include infective causes such as encephalitis, Acute Disseminated Encephalomyelitis (ADEM), other drugs such as immunosuppressive agents like cyclosporine or antimicrobial agents such as amphotericin B. Drugs of abuse such as methanol, cocaine and heroin are also known to cause encephalopathy changes. Therefore, MRI features of cerebral white matter T2/FLAIR hyperintensity along with a thorough clinical history pointing to the intake of specific toxic entity, is essential to make a diagnosis [1,3].
In the first case of our series who was being treated with 5-FU, we found restricted diffusion with only subtle findings on conventional MRI, similar to the findings of Akitake R et al., in a patient treated by 5-FU for esophageal cancer [5]. These findings suggested an acute presentation of disease which was resolved on further imaging. A complete reversal of clinico-radiological encephalopathic findings in a case of carcinoma rectum being treated on 5-FU was noted by Raut TP et al., [10].
In our second case on long term methotrexate therapy, we found MRI changes on only FLAIR images and no diffusion restriction. This patient had been on a long term therapy of methotrexate since six months and it could be deduced that the patient was on recovery course from toxic changes where diffusion changes had already reversed and conventional MRI findings were still present; similar to the observations of Salkade PR et al., [8].
There is no definite treatment for toxic leukoencephalopathy. Immediate stoppage of offending agent along with supportive therapy with corticosteroids, antioxidants such as coenzyme Q, vitamin E and vitamin C may lead to clinico-radiological improvement over time in reversible cases, but still lack substantial research back up [11,12].
Conclusion
A prompt clinical observation of a new onset neurological deficit with aid of conventional and DWI can help in early diagnosis which may reverse and further prevent the neurological manifestations.
[1]. Filley CM, Kleinschmidt-DeMasters BK, Toxic leukoencephalopathyNew England Journal of Medicine 2001 345(6):425-32. [Google Scholar]
[2]. Sioka C, Kyritsis AP, Central and peripheral nervous system toxicity of common chemotherapeutic agentsCancer Chemotherapy and Pharmacology 2009 63(5):761-67. [Google Scholar]
[3]. McKinney AM, Kieffer SA, Paylor RT, SantaCruz KS, Kendi A, Lucato L, Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imagingAmerican Journal of Roentgenology 2009 193(1):192-206. [Google Scholar]
[4]. Rollins N, Winick N, Bash R, Booth T, Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcomeAmerican Journal of Neuroradiology 2004 25(10):1688-95. [Google Scholar]
[5]. Akitake R, Miyamoto SI, Nakamura F, Horimatsu T, Ezoe Y, Muto M, Early detection of 5-FU-induced acute leukoencephalopathy on diffusion-weighted MRIJapanese Journal of Clinical Oncology 2011 41(1):121-24. [Google Scholar]
[6]. McKendry RJ, The remarkable spectrum of methotrexate toxicitiesRheumatic Disease Clinics of North America 1997 23(4):939-54. [Google Scholar]
[7]. Sivasubramanian S, Moorthy S, Sreekumar KP, Kannan RR, Diffusion-weighted magnetic resonance imaging in acute reversible toxic leukoencephalopathy: A report of two casesIndian Journal of Radiology and Imaging 2010 20(3):192-94. [Google Scholar]
[8]. Salkade PR, Lim TA, Methotrexate-induced acute toxic leukoencephalopathyJournal of Cancer Research and Therapeutics 2012 8(2):292-96. [Google Scholar]
[9]. Yagmurlu B, Akyürek S, Fitoz S, Demirkazik A, MRI of non-neoplastic cranial complications of malignant disordersDiagnostic and Interventional Radiology 2008 14(2):61-68. [Google Scholar]
[10]. Raut TP, Mathur T, Ambulkar I, Gupta K, Ansari K, Janga S, Severe reversible form of chemotherapy induced acute disseminated leuko-encephalopathy: a case report with review of literatureAnn Clin Lab Res 2017 5:1 [Google Scholar]
[11]. Kriegstein AR, Shungu DC, Millar WS, Armitage BA, Brust JC, Chillrud S, Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”)Neurology 1999 53(8):1765-73. [Google Scholar]
[12]. Sanaei-Zadeh H, Toxic leukoencephalopathyMOJ Toxicol 2016 2(2):33 [Google Scholar]