Renal Synovial Sarcoma in a Young Pregnant Lady: A Case Report and Clinico-Pathological Profile
Gregory Pathrose1, Nirmal Thampi John2, Pradeep Hariharan3
1 Assistant Professor, Department of Urology, Mar Baselious Medical Mission, Ernakulam, Kerala, India.
2 Professor, Department of Urology, Christian Medical College, Vellore, Tamil Nadu, India.
3 Assistant Professor, Department of Pathology, Christian Medical College, Vellore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nirmal Thampi John, Professor, Department of Urology, Christian Medical College, Vellore-632002, Tamil Nadu, India E-mail : nirmaltj@gmail.com
Synovial sarcoma is a soft tissue neoplasm with clearly defined histologic, immunohistochemical and molecular features. These tumours usually arise in the extremities of young adults. Their occurrence in the kidney is extremely rare. A 25-year-old pregnant lady in her first trimester was incidentally found to have a left renal mass on perinatal ultrasonography. MRI showed a well encapsulated, heterointense mass replacing the left kidney. Following medical termination of her pregnancy, a radical nephrectomy was performed. Histopathology revealed a primary synovial cell sarcoma of the kidney. Postoperatively, she received ifosfamide based adjuvant chemotherapy. This report highlights the challenges involved in the diagnosis of this extremely rare neoplasm. A high index of clinical suspicion, complimented by the use of immunohistochemistry and cytogenetics during histopathological analysis aide in the diagnosis. Aggressive management with a combination of complete surgical extirpation and chemotherapy gives the best results.
Chemotherapy, Cytogenetics, Immunohistochemistry
Case Report
A 25-year-old pregnant lady (G2P1L1) at 10+4 weeks gestation was incidentally diagnosed to have a left renal mass on perinatal USG.
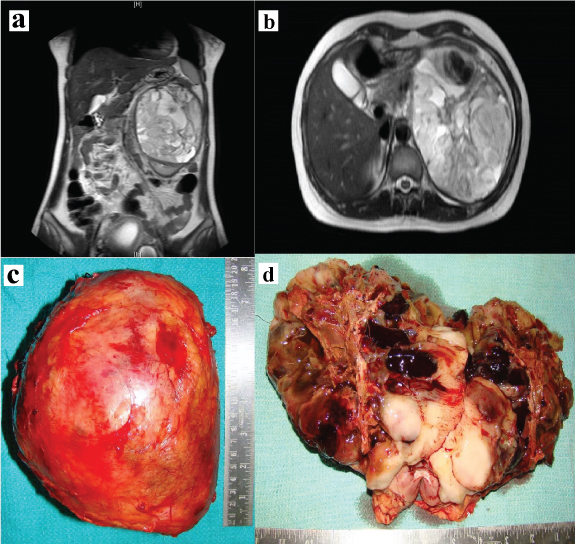
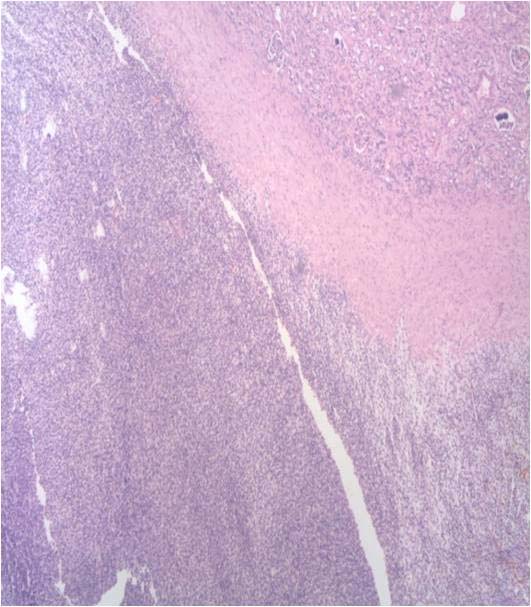
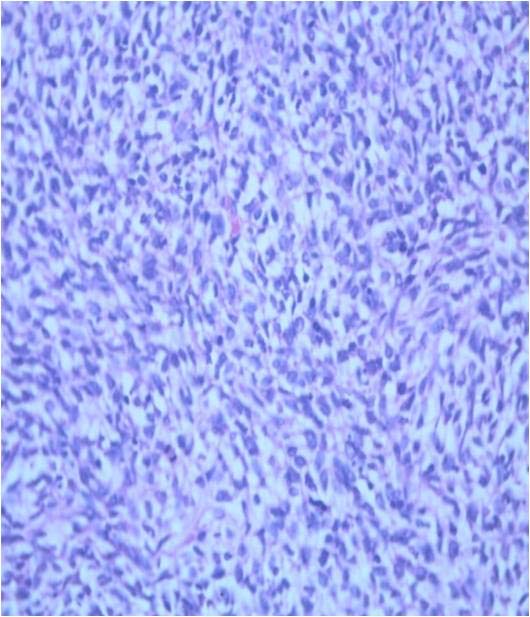
She had no haematuria, bone pain, jaundice, loss of appetite or loss of weight. There was a palpable left loin mass which was ballotable and bimanually palpable. MRI abdomen revealed a 13 x 11 x 10 cm hetero-intense mass arising from the left kidney with effacement of pelvi-calyceal systems and foci of haemorrhage suggestive of renal cell carcinoma [Table/Fig-1a,b]. There was no obvious hilar or para-aortic lymphadenopathy. Following termination of pregnancy, she underwent an open radical nephrectomy. Gross examination showed the mass to be well encapsulated [Table/Fig-1c]. The tumour had completely replaced the left kidney and cut section revealed cystic spaces with areas of necrosis and haemorrhage [Table/Fig-1d]. Microscopic examination showed renal parenchyma with a well circumscribed cellular neoplasm composed of round to oval to spindle shaped cells in sheets, short fascicles and focal perivascular arrangement [Table/Fig-2]. The tumour cells had scanty eosinophilic cytoplasm and hyperchromatic mitotically active nuclei, including a few with prominent nucleoli [Table/Fig-3]. There were large areas of necrosis along with foci of myxoid change.
a,b) MRI T2W, coronal and axial cuts showing a hetero-intense left renal mass; c,d) A well encapsulated mass with cut section showing cystic areas with necrosis and haemorrhage.
Cellular tumour with areas of necrosis, H&E x200.
Spindle shaped cells in the tumour, H&E x400.
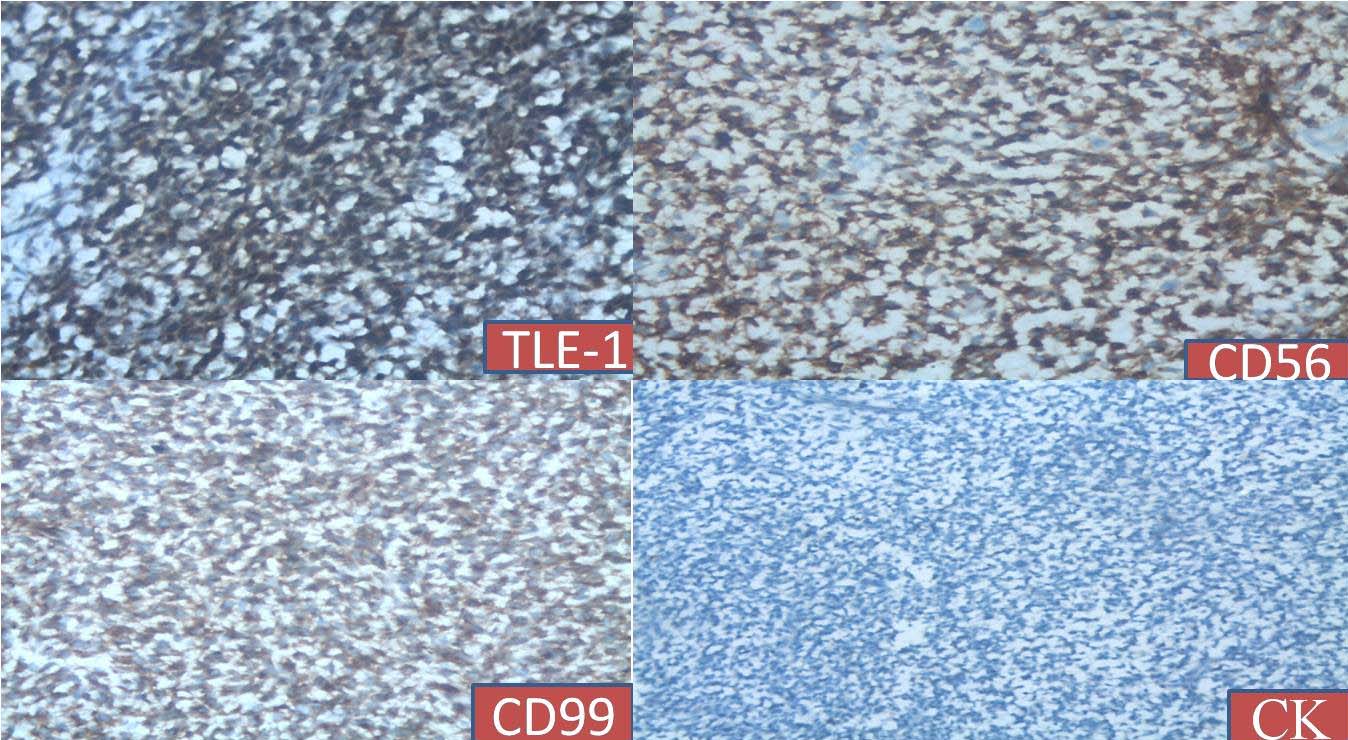
On immunohistochemistry the tumour cells were positive for TLE-1, CD56, CD99 [Table/Fig-4] and vimentin and negative for desmin, myogenin, EMA, CK and WT1. With this profile a diagnosis of synovial sarcoma was suggested. Subsequent molecular analysis showed the presence of SYT-SSX2 translocation which was confirmatory for the same.
IHC panel done on the tumour showing immune-positivity for TLE-1, CD56, CD99 and immune-negativity for CK; IHC x400.
The sampled lymph nodes did not show evidence of metastasis. Although this patient received a R0 resection, she was given adjuvant chemotherapy with three cycles of ifosfamide (1800 mg/m2) and doxorubicin (25 mg/m2) considering the aggressive pathology and remained disease free at follow up of three years.
Written informed consent was obtained from the patient for publication of this case report and all accompanying images.
Discussion
Synovial cell sarcoma was first described by Argani P et al., in 2000 [1]. It accounts for 5%-10% of sarcomas in adolescents and young adults after liposarcoma and Malignant Fibrous Histiocytoma (MFH) [2]. Synovial sarcoma occurs primarily in the soft tissues and is a translocation associated mesenchymal neoplasm occurring mostly in para-articular regions of the extremities [2]. They are also found in rare sites like head and neck, thorax and retroperitoneum. These tumours have also been described in several unusual locations, including the pleura, lung and mediastinum. However, renal synovial sarcomas are extremely rare. This case is the first documented in a young pregnant lady.
They usually arise from pleuripotent mesenchymal cells and are morphologically monophasic consisting of only spindle cells or biphasic with both epithelial and spindle cells on microscopy [1]. The gross picture of the tumour is represented by thick walled cystic and necrotic areas. The lining cells are eosinophilic, polygonal with apically placed nuclei (hobnail nuclei).
Synovial sarcomas are classified as monophasic, biphasic and poorly differentiated. Synovial sarcomas on immunohistochemistry show positive staining for EMA in both epithelial and spindle cell component [3].
In around 30% of cases synovial sarcomas stain positive for S-100 and in two thirds of cases CD99 positivity is also present. Among cytokeratins, CK7 and CK19 are frequently positive in synovial sarcomas. Synovial sarcomas show immune-negativity for CD34 which helps distinguish from solitary fibrous tumour [3].
Monophasic/well differentiated synovial sarcoma of the kidney shows considerable morphologic overlap with other renal spindle cell tumours especially sarcomatoid renal cell carcinoma, cellular mesoblastic nephroma and embryonal rhabdomyosarcoma; whereas poorly differentiated synovial sarcoma of the kidney can be difficult to distinguish from other round cell sarcomas, especially Ewing sarcoma/ Primitive Neuro Ectodermal Tumour (PNET), adult blastemal Wilms tumour and alveolar rhabdomyosarcoma [4]. These differential diagnoses in this case were ruled out by immune-negativity for the relevant markers.
TLE-1 expression is a consistent and prominent feature of synovial sarcoma and TLE-1 is a sensitive and highly specific marker for synovial sarcoma and helps to differentiate synovial sarcoma from other histological mimics. Cytogenetically monophasic, biphasic and poorly differentiated forms show a reproducible tumour specific translocation, t(X;18)(p11.2;q11.2), which results in the production of one or the other principal fusion genes, SYT-SSX1 and SYT-SSX2. Monophasic forms show SYT-SSX2 and biphasic forms show SYT- SSX1 fusion. Hence the use of immunohistochemistry and RT-PCR for finding SYT-SSX fusion genes which is the gold standard was done in the present case and is necessary to arrive at the correct diagnosis [3-5].
Analysis of the clinical features of reported cases of primary renal synovial sarcoma shows that it occurs in adults with a mean age of 38.5 (range: 20-59) years. It has a male predominance with a male-to-female ratio of 1.7:1. Patients usually present with local pain or haematuria. The tumour is often large at the time of diagnosis, ranging in size from 5 to 19 cm with a mean size of 10 cm as in the present case [6]. On CT imaging, synovial sarcomas appear heterogenously enhancing with areas of calcification and air fluid levels. On MRI, a distinct ‘triple sign’ is seen which represents areas of calcification, haemorrhage and air-fluid levels [7].
Most of the synovial sarcomas are aggressive with five year mortality rates of 25% following resection [8]. Their management entails complete surgical extirpation as positive surgical margins on histology confers a poor prognosis [9]. Tumour size and SYT-SSX translocation are independent predictors of prognosis with tumours more than 5 cm and SYT-SSX1 mutations having worst outcomes [10].
Moreover, 30% to 50% of the patients develop distant metastasis despite adequate resection. Adjuvant chemotherapy with ifosfamide and doxorubicin has been reported to enhance survival [9].
Conclusion
This report highlights the challenges encountered in the diagnosis and management of primary renal synovial sarcoma. Despite being extremely uncommon, it should be included in the differential diagnosis of benign and malignant spindle cell tumours of the kidney. Aggressive management using a combination of surgery and chemotherapy confers the best results.
[1]. Argani P, Faria PA, Epstein JI, Reuter VE, Perlman EJ, Beckwith JB, Primary renal synovial sarcoma: molecular and morphological delineation of entity previously included among embryonal sarcomas of the kidneyAm J Surg Pathol 2000 24(8):1087-96. [Google Scholar]
[2]. Spillane AJ, A’Hern R, Judson IR, Fisher C, Thomas JM, Synovial sarcoma: a clinicopathologic, staging and prognostic assessmentJ Clin Oncol 2000 18(22):3794-803. [Google Scholar]
[3]. Fletcher CDM, Diagnostic histopathology of tumours 2013 4th edChurchill Livingstone:1847-49. [Google Scholar]
[4]. Wang J, Weiss LM, Hu B, Chu P, Zuppan C, Felix D, Usefulness of immunohistochemistry in delineating renal spindle cell tumours. A retrospective study of 31 casesHistopathology 2004 44(5):462-71. [Google Scholar]
[5]. Foo WC, Cruise MW, Wick MR, Hornick JL, Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimicsAm J of Clin Pathol 2011 135(6):839-44. [Google Scholar]
[6]. Chen S, Bhuiya T, Liatsikos EN, Alexianu MD, Weiss GH, Kahn LB, Primary synovial sarcoma of the kidney, a case report with literature reviewInt J Surg Pathol 2001 9(4):335-39. [Google Scholar]
[7]. Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C, Pediatric and adult sarcomas of the kidney: a cross sectional imaging reviewActa Radiol 2011 52(4):448-57. [Google Scholar]
[8]. Lacovelli R, Altavilla A, Ciardi A, Urbano F, Manai C, Gentile V, Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literatureBJU Int 2012 110(10):1449-54. [Google Scholar]
[9]. Devita VT, JrHelman S, Rosenberg SA, Cancer principles & practice of oncology 2001 6th edPhiladelphiaLippincott Williams & Wilkins:1841-73. [Google Scholar]
[10]. Trassard M, Le DV, Hacene K, Terrier P, Ranchère D, Guillou L, Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patientsJ Clin Oncol 2001 19(2):525-34. [Google Scholar]