Intensive Care Units (ICU) are the most frequently identifiable source of hospital acquired infections with several fold higher infection rates than general hospital wards. Patients admitted to the ICU are at a higher risk of developing hospital acquired infections. Widespread and injudicious use of broad spectrum antimicrobials in the ICUs has led to the emergence of several resistant strains of microbes which contributes significantly to raise the health care costs and also patient morbidity and mortality [1-4].
Rising concerns about antimicrobial resistance and inadequate development of effective new anti-infective drugs have stimulated universal efforts to strengthen infection-control interventions and antimicrobial stewardship practices [5-8]. Antimicrobial stewardship is a rational, systematic approach to promote the optimal selection, dosing, and duration of therapy for antimicrobial agents throughout the course of their use in order to improve the outcomes. Successful stewardship programs have improved antimicrobial prescribing practices in terms of optimal selection, dose, duration, and route of therapy, decreased antimicrobial use and resistance, reduced unnecessary pharmacy expenditures [9-11].
The literature suggests that ASPs are associated with reduced ICU antimicrobial utilization [12]. The recommendations to modify utilization of antimicrobials may not be applicable to all hospitals, as the spectrum of potential pathogens, patients at risk of infection are unique to individual hospitals. There is limited information regarding studies on change in prescribing pattern of antimicrobials after implementation of ASP done in Indian hospitals.
In SKH, antibiotic stewardship program was first implemented in 2013 as a crucial step to improve antimicrobial prescribing practices in accordance with antibiotic policy. This study was planned to assess the change in antimicrobial use before and after implementation of ASP i.e., in the years 2012 and 2015; and to study the rate and pattern of antimicrobial use in medical ICU.
Materials and Methods
This was a cross-sectional, observational study conducted in 12 bedded Medical Intensive Care Unit (MICU), over a period of two years from October 2014 to October 2016 at Shree Krishna Hospital and Medical Research Centre, a tertiary care teaching rural hospital attached to Pramukhswami Medical College, Karamsad, Gujarat, India. Approval was taken from Institutional Human Research Ethics Committee. Confidentiality of all participants was maintained at all levels.
Sample size was calculated with the help of Winpepi software. The estimated sample size was 206 at 5% significance level and 80% power but due to feasibility issue, data were collected from 150 case files. A total of 150 case files i.e., 75 from year 2012 and 75 from year 2015 were retrieved from medical record section of the hospital. Data was collected retrospectively over a period of six months i.e., January 2015 to June 2015. Patients on anti-bacterial drugs admitted in medical ICU were included while patients on anti-fungal, anti-viral, anti-tubercular, anti-leprosy drugs were excluded. Prior permission was taken from the system department of the hospital to get the file numbers of patients admitted to Medical ICU. Since the data were collected from medical record section of hospital, waiver from informed consent form was obtained.
Appropriateness of prescriptions was decided on the basis of appropriateness of choice, dose, frequency and duration of antimicrobial agents. Appropriateness of these parameters was decided by referring standard text books like Harrison’s Principles of Internal Medicine [13] and Goodman and Gilman’s Pharmacological basis of Therapeutics [14]; National Standard Treatment Guidelines and Antibiotic Policy of SKH.
Statistical Analysis
Descriptive statistics was used to find out frequency of different variables and rate of various antimicrobial agents prescribed in MICU. Chi-square test was applied to compare the prescribing pattern of antimicrobials pre and post implementation of ASP i.e., between the years 2012 and 2015. Results were considered statistically significant if p-value < 0.05.
Results
Total 150 case files were studied from MICU of SKH, 75 case files from year 2012 and 75 case files from year 2015. Out of 150 patients, there were 103 (68.67%) males and 47 (31.33%) females. Minimum age was 20 years and maximum age was 91 years. The mean (±SD) age was 57.11 (±16.83) years. Majority of the patients i.e., 41 (54.67%) in year 2012 and 45 (60%) in year 2015 were suffering from respiratory conditions. Most of the patients admitted in MICU were from general medicine and chest medicine. Patients’ characteristics are mentioned in [Table/Fig-1]. Culture sensitivity test was done in 39 cases of 2012 and 28 cases of 2015, while this test was not done in rest of the cases [Table/Fig-2].
Characteristics of the patients admitted in MICU.
| Patient characteristics | MICU |
|---|
| 2012 (n=75) | 2015 (n=75) |
|---|
| Gender |
| Male | 50 (66.67%) | 53(70.33%) |
| Female | 25 (33.33%) | 22(29.33%) |
| Age groups (years) |
| 18-40 | 16 (21.33%) | 10 (13.33%) |
| 41-65 | 35 (46.67%) | 41(54.67%) |
| >65 | 24 (32%) | 24 (32%) |
| Mean (±SD) ICU stay | 3.03 (±1.93) | 3.28 (±1.79) |
| Outcome |
| Discharge | 46 (61.33%) | 50 (66.67%) |
| Discharge Against Medical Advice (DAMA) | 24 (32%) | 20 (26.67%) |
| Death | 5 (6.67%) | 5 (6.67%) |
Culture sensitivity tests of the patients admitted in MICU.
| Culture sensitivity reports | MICU |
|---|
| 2012 (n=39) | 2015 (n=28) |
|---|
| During ICU stay | 35 | 23 |
| Outside the ICU stay | 4 | 5 |
| Result of C/S tests | 2012 (n=39) | 2015 (n=28) |
| Positive | 19 | 10 |
| Negative (no organism) | 20 | 18 |
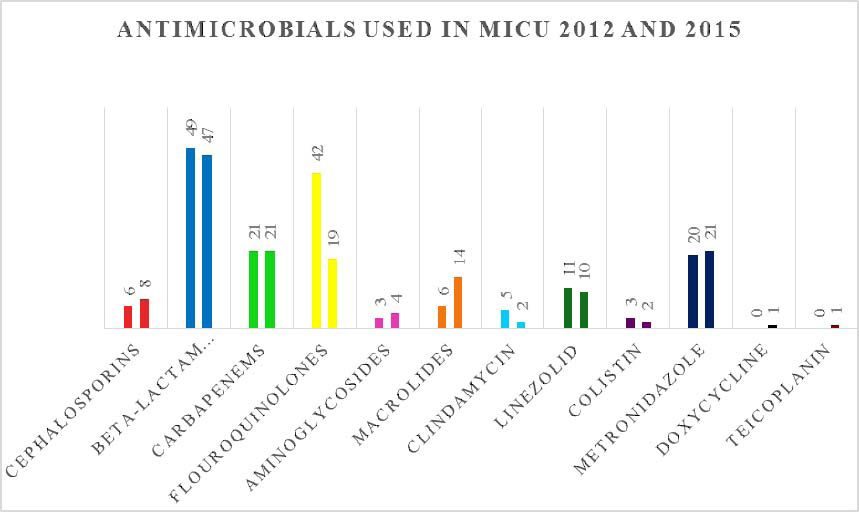
Characteristics of antimicrobial drugs prescribed in MICU, are mentioned in [Table/Fig-3]. The most common group of drugs prescribed in MICU was β-lactam antibiotics + β-lactamase inhibitors during 2012 as well as 2015, where in piperacillin + tazobactam and amoxicillin + clavulanic acid were commonly prescribed in 2012 whereas cefepime + tazobactam was commonly prescribed in 2015 [Table/Fig-4,5]. Total 96 fixed dose combinations were prescribed in MICU during both the years [Table/Fig-6].
Characteristics of antimicrobial drugs prescribed in MICU.
| Antimicrobial drugs | MICU |
|---|
| 2012 | 2015 |
|---|
| 1. Mean (SD) | 2.21 (0.67) | 2 (0.76) |
| 2. Prescribed by- |
| Generic name | 77 | 106 |
| Brand name | 89 | 44 |
| 3. Appropriateness of antimicrobial drug regimen |
| Empirical |
| Choice of antimicrobial drug | 54 | 66* |
| Dose | 66 | 70 |
| Frequency | 66 | 70 |
| Duration | 47 | 47 |
| Prophylactic |
| Choice | 1 | 5* |
| Dose | 6 | 5 |
| Frequency | 6 | 5 |
| Duration | 2 | 1 |
* p-value < 0.05
List of prescribed antimicrobial groups/drugs in MICU.
| Antimicrobial drugs | MICU |
|---|
| 2012 | 2015 |
|---|
| Cephalosporins | 6 (3.61%) | 8 (5.33%) |
| Ceftriaxone | 1 | 5 |
| Cefazolin | 0 | 0 |
| Cefotaxime | 3 | 1 |
| Cefuroxime | 2 | 1 |
| Cefepime | 0 | 1 |
| β-lactam antibiotics + β-lactamase inhibitors | 49(29.51%) | 47(31.33%) |
| Piperacillin + Tazobactam | 26 | 9 |
| Amoxicillin + clavulanic acid | 20 | 7 |
| Ampicillin + sulbactam | 1 | 1 |
| Cefepime + Tazobactam | 2 | 30 |
| Cefoperazone + sulbactam | 0 | 0 |
| Carbapenems | 21(12.65%) | 21 (14%) |
| Meropenem | 7 | 20 |
| Imipenem | 14 | 0 |
| Doripenem | 0 | 1 |
| Fluoroquinolones | 42 (25.3%) | 19(12.67%) |
| Levofloxacin | 35 | 16 |
| Ofloxacin | 6 | 3 |
| Ciprofloxacin | 1 | 0 |
| Aminoglycosides | 3 (1.8%) | 4 (2.67%) |
| Amikacin | 3 | 4 |
| Gentamicin | 0 | 0 |
| Macrolides | 6 (3.61%) | 14 (9.33%) |
| Clarithromycin | 5 | 14 |
| Azithromycin | 1 | 0 |
| Clindamycin | 5 (3%) | 2 (1.33%) |
| Linezolid | 11 (6.62%) | 10 (6.67%) |
| Colistin | 3 (1.8%) | 2 (1.33%) |
| Metronidazole | 20(12.05%) | 21 (14%) |
| Doxycycline | 0 | 1 (0.67%) |
| Teicoplanin | 0 | 1 (0.67%) |
| Tigecycline | 0 | 0 |
| Total | 166 | 150 |
Distribution of prescribed antimicrobial groups/drugs in MICU 2012 and 2015.
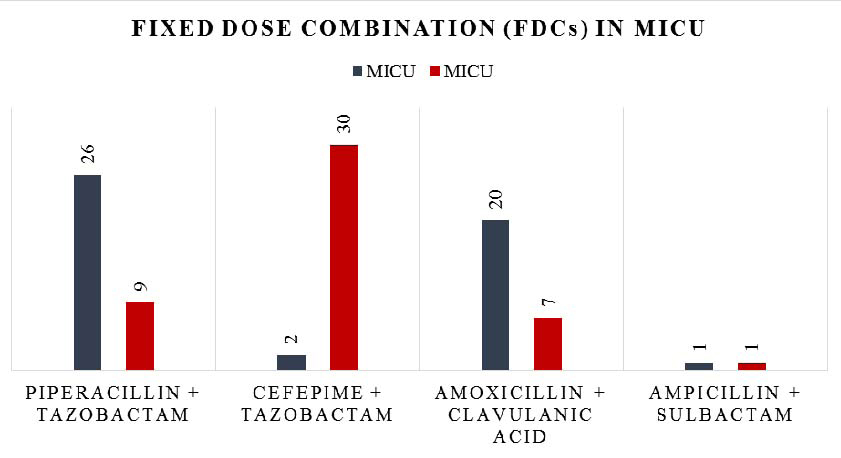
Use of Fixed Dose Combination (FDCs) in MICU before and after implementation of ASP.
Overall rate of prescribing restricted antimicrobials in MICU during 2015 was 36% (54/150) which includes meropenem as most common followed by linezolid, piperacillin + tazobactam and others [Table/Fig-7].
List of restricted antimicrobial drugs in year 2015.
| Restricted antimicrobial drugs | MICU2015 |
|---|
| Cefuroxime | 1 |
| Ceftriaxone | 5 |
| Meropenem | 20 |
| Imipenem | 0 |
| Piperacillin - tazobactam | 9 |
| Teicoplanin | 1 |
| Colistin | 2 |
| Amikacin | 4 |
| Linezolid | 10 |
| Clindamycin | 2 |
| Total | 54 |
Most of the patients (i.e., 146) received medicines by intravenous route. Only four out of 150 patients were given medicines by oral route. Four patients from 2012 and two patients from 2015 were switched over to oral route from parenteral (IV) route. All the antimicrobials switched to oral route were appropriate.
In MICU, total 139 patients i.e., 69 (92%) patients in 2012 and 70 (93.33%) patients in 2015 were given antimicrobial for therapeutic purpose. Out of 69 patients of 2012, 66 patients were treated empirically and three patients were given definite treatment while all 70 patients of 2015 were treated empirically. Only 11 patients of MICU i.e., 6 (8%) from 2012 and 5 (6.67%) from 2015 were given prophylactic antimicrobials for the conditions like thalamic bleed, intracranial hemorrhage, blast crisis and spontaneous abortion. Total duration of prophylaxis was 26 days with an average of 4.33 days per patient in 2012 and 29 days with an average of 5.8 days per patient in 2015. Antimicrobials were escalated in 11 (14.67%) of 2012 while in 7 (9.33%) cases of 2015; and de-escalated in only 2 (2.67%) cases from 2012 as well as 2015.
In 58 (77.33%) prescriptions of 2012 and 71 (94.67%) prescriptions of 2015, choice of antimicrobial drugs was appropriate (p-value=0.008). There was no need to give antimicrobials in 5 (6.67%) prescriptions of 2012 and 2 (2.67%) prescriptions of 2015. Dose and frequency of antimicrobial drugs were appropriate in all prescriptions (100%) of MICU in 2012 as well as of 2015. In 50 (66.67%) prescriptions of 2012 and 48 (64%) prescriptions of 2015, duration of antimicrobial drugs was appropriate. Appropriateness of duration was not applicable in 22 (29.33%) prescriptions of 2012 and 23 (30.67%) prescriptions of 2015 in MICU. Appropriateness of empirical and prophylactic antimicrobial drug regimens is mentioned in [Table/Fig-3].
In MICU, 57 (76%) antimicrobial prescriptions of 2012 were matching to antibiotic policy of SKH and 67 (89.33%) antimicrobial prescriptions of 2015 were adhering to antibiotic policy of SKH. This difference is statistically significant, p-value=0.031.
Discussion
In present study, many changes were observed related to utilization pattern of antimicrobials in MICU as well as SICU during 2015 as compared to 2012.
As reported by study done by Diaz Granados C [15], the current study also showed gender distribution with male preponderance (>68%) in MICU during 2012 as well as 2015. Out of 150 patients, 76 (50.67%) were from the age group of 41-65 years. In current study, the mean (±SD) age was 57.11 years (±16.83); which is lower than the study done by Badar VA et al., i.e., 50 years [16].
In MICU, maximum patients i.e. 41 (54.67%) during 2012 and 45 (60%) during 2015, were suffering from respiratory conditions like exacerbation of Chronic Obstructive Pulmonary Disease (COPD) and aspiration pneumonia as common LRTIs. The study done by Banerjee T et al., showed that 35.29% were diagnosed with respiratory infections [17].
In the current study, mean (±SD) MICU stay was 3.03 (±1.93) days in 2012 and 3.28 (±1.79) days in 2015. These mean lengths of stay were found shorter than 5.67 days per medical patient, shown in the study done by Williams A et al., [18].
Culture sensitivity tests were done in 39 (52%) cases during 2012 and 28 (37.3%) cases during 2015. It was observed that more than half of the culture reports of this study were negative as they did not show growth of any pathogen. In a study done by Banerjee T et al., microbiological investigations had been sent for only in 51.17% of the patients following admission to the ICU where majority of the cases (60/87) were culture positive [17]. Many a times patients come to tertiary care centers after taking prior medications at the previous hospitals so culture sensitivity reports may not be very informative for the clinicians who may not prefer to wait for results of culture sensitivity. This may contribute to the lower trend of culture sensitivity tests in current study.
Mean (±SD) number of antimicrobials per patient was 2.1 (±0.73) in present study, similar to the study done by Williams A et al., where in a mean (±SD) of 2.09 (±1.27) antibiotics/prescription has been reported [18]. Study done by Hanssens Y et al., reported an average of almost three antibiotics/prescription in MICU [19]. A study conducted in Southern India reported the mean of 1.60 (±0.77) antibiotics/prescription which is lower than current study [20].
In MICU, majority of antimicrobial drugs were prescribed by brand name during 2012 but the ratio of brand to generic got reversed during 2015 as majority of antimicrobial drugs were prescribed by generic name. A study done by Pandiamunian J et al., reported 29.20% antimicrobials were prescribed by generic names and the remaining 70.79% were prescribed by brand names [21]. According to the recent gazette notification by MCI, clinicians are advised to prescribe the drugs by generic name [22]. The rates of prescribing medicines by generic name are expected to be improved with this. Many times clinicians strongly prefer particular brands as they do not want to do any compromise in efficacy of antimicrobials in ICU setups.
The most common group of drugs prescribed in MICU was β-lactam antibiotics + β-lactamase inhibitors (almost 30% of all antimicrobials) during 2012 and 2015. Prescribing rate of fluoroquinolones, clindamycin, linezolid and colistin had decreased during 2015 as compared to 2012. Prescribing rate of macrolides, cephalosporins, carbapenems, aminoglycosides and metronidazole had increased during 2015 as compared to 2012. Decrease in the prescribing rate of piperacillin + tazobactam and imipenem in 2015 highlight the impact of ASP as these drugs are listed under heading of restricted antimicrobials in antibiotic policy.
In a study conducted by Patel MK et al., cephalosporins, metronidazole and penicillins were the most commonly prescribed antimicrobials in critical care unit [23]. Metronidazole was most commonly prescribed followed by ceftriaxone in a study conducted in Western Nepal [24]. In a study conducted during 2013, fluoroquinolones were prescribed in 8.8% and doxycycline was prescribed in 0.9% of prescriptions of MICU [21]. Another study reported utilization rates of linezolid 1% and clindamycin 3.8% in MICU [25]. We could not find the studies to compare utilization of each antimicrobial agent in MICU.
‘Restricted antibiotics’ refer to those antibiotics that can contribute to development of multi-resistant organisms [26]. These are antimicrobial agents that can be used in accordance with restrictions mentioned in antibiotic policy. The use of ‘restricted antibiotics’ could be done by determining the infection and risk stratification in the patient; and sending a sample for culture and sensitivity. In this study, patient risk stratification was not mentioned in any of the total case files. In current study, overall rate of prescribing restricted antimicrobials in MICU during 2015 was 36% which includes meropenem as most common followed by linezolid, piperacillin + tazobactam and others.
Total 96 FDCs were prescribed in MICU where piperacillin + tazobactam were the highest prescribed FDC followed by amoxicillin + clavulanic acid during 2012 while cefepime + tazobactam were the highest prescribed FDC during 2015. A study conducted by Patel MK [23], reported piperacillin + tazobactam was the highest prescribed FDC in critical care unit. While cefoperazone+sulbactam (30.8%) were the most common FDC prescribed in MICU in a study conducted by John LJ et al., [25].
In current study, 92% patients in 2012 and 93.33% patients in 2015 were given antimicrobial for therapeutic purpose; out of which most of the patients were treated empirically. This is comparable to the result given by Badar VA et al., wherein antimicrobials were given for therapeutic purpose in 89% and for prophylactic purpose in 11% of patients [16]. In this study, mean duration of prophylaxis in MICU was 4.33 days per patient in 2012 and 5.8 days per patient in 2015. In a study conducted by Agrawal A et al., in same institute, the mean duration of antimicrobial prophylaxis in MICU was 2.58 days [27].
Escalation of antimicrobial agents was done in only 19 (12.67%) while de-escalation of antimicrobial agents was done in only 4 (2.67%) out of 150 prescriptions. The study conducted by Malacarne P et al., reported that antibiotic therapy was escalated in 37.6% of patients and de-escalated in 24% of patients of ICU [28]. Comprehensive ASPs have demonstrated reduction in antimicrobial use, institutional antimicrobial resistance rates and hospital cost [29].
According to the findings of current study, appropriateness of antimicrobial choice had improved from 77.33% in year 2012 to 94.67% in year 2015 in MICU (p-value=0.008). Statistically significant improvement was observed in appropriateness of choice prophylactic antimicrobials (p-value=0.022) as well as empirical antimicrobials (p-value=0.040) in MICU during 2015. Dose and frequency of antimicrobial drugs were appropriate in all prescriptions (100%) of MICU in 2012 as well as of 2015. Duration of antimicrobial drugs was appropriate in 66.67% prescriptions of 2012 and 64% prescriptions of 2015.
According to the findings of this study, appropriateness of prescriptions had significantly improved from 76% of 2012 to 89.33% in 2015 in MICU (p-value=0.031). In a study conducted by Amer MR et al., the ASP implementation in medical ICU improved appropriateness of empirical antibiotics utilization from 30.6% in the historical control arm to 100% in the proactive ASP arm [30].
Recovery rates had increased from 61.33% to 66.67% in MICU during 2015 as compared to 2012 with decrease in the rates of DAMA. Death rates were same during 2012 as well as in 2015 (6%). The death rates of current study were very low as compared to a study conducted by Williams A et al., where 60.5% patients were discharged from the ICU after recovery while 39.5% patients were expired [18].
We could not include all the patients from 2012 and 2015 who were admitted in MICU and prescribed antimicrobials. Duration of 45 patients could not be analysed due to their DAMA. So the appropriateness of antimicrobials prescribed in these patients was decided on the basis of choice, frequency and dose of the drugs. Majority of patients did not undergo culture sensitivity reports, so the escalation or de-escalation of antimicrobials was done on the basis of prognosis of patient in terms of deterioration or improvement. However, above limitations do not affect the importance of key findings of this study.
Conclusion
In-depth analysis of the study revealed a positive impact of ASP and antibiotic policy. Implementation of ASP in year 2013, brought an effective increase in the appropriate use of antimicrobials.
* p-value < 0.05