The Parafollicular (PF) cell is present in the thyroid gland and is a part of neuroendocrine system. Based on histochemical properties, they have been classified as APUD cells [1]. They have been called by variety of names for example- C cell, neurohormonal cell, giant-light cell, argyrophil cell, light cell and mitochondria-rich cell by various researchers. They secrete a hormone ‘calcitonin’ which is involved in maintaining calcium homeostasis [2].
Histologically, the thyroid gland is formed by follicular and PF cells. Even though they share same location, these cells differ considerably in terms of origin, morphology and physiological role. The former are the predominant cells which are endodermally derived and arranged in the form of follicles. They are engaged in the production and release of tetra-iodothyronine (thyroxine or T4) and tri-iodothyronine (T3). On the other hand, PF cells are neural crest cells derivative and colonize mainly the middle third of each lateral lobe of the thyroid gland. They are typically found scattered within thyroid follicles, lying inside the basement membrane but not reaching the follicular lumen, which is called as an intrafollicular position. Occasionally, they occur in clusters in the interfollicular connective tissue stroma, which is called as a parafollicular/interfollicular position [3,4].
Due to its location, the growth and development of PF cells may be closely related to the maturation of thyroid follicles. This is a base line study attempting to describe the sequential development of PF cells with a particular focus on its correlation with developing human thyroid follicles.
Materials and Methods
A descriptive study was conducted between September 2013 and April 2014 on 10 foetuses of gestational age 14-28 weeks procured from Department of Obstetrics and Gynaecology, Lok Nayak Hospital, New Delhi. After obtaining approval from the Institutional Ethical Committee of Maulana Azad Medical College and associated Lok Nayak Hospital, the sample size was determined by the availability of foetuses. Informed consent of parents was taken and patient secrecy was conserved. Foetuses below the gestational age of 20 weeks were obtained from abortions conducted in accordance with the Medical Termination of Pregnancy Act 1954, while those above 20 weeks of gestation was obtained from stillbirths. A detailed maternal history was recorded. An initial assessment of the foetus was done to exclude out any gross abnormality. The gestational ages of the foetuses was determined by measuring Crown-Rump Length (CRL), Biparietal Diameter (BPD), Foot Length (FL) and weight of foetus. Incision was given longitudinally on the anterior aspect of neck in the median plane for better penetration of the fixative into the neck. The foetus was then fixed by immersion in 10% buffered neutral formalin solution. After a week of fixation, the thyroid gland was dissected. The right lobe was arbitrarily preferred and preserved in fresh fixative for another one week. The specimens were labelled and processed for paraffin embedding. About 6 μ-thick serial sections were generated on a rotary microtome with the lateral surface of the right lobe of thyroid gland as the cutting surface.
Serial sections were stained with Haematoxylin and Eosin (H&E) stain. The entire series was examined for PF cells and morphology of thyroid gland. In each foetal thyroid, every tenth section was processed for Immunohistochemistry (IHC). Deparaffinised sections were incubated in citrate buffer and the endogenous peroxidase action was blocked using methanol and 1% H2O2. After washing with working solution of phosphate buffer with 0.1 Triton-X, slides were treated with normal horse serum for two hour for blocking the non-specific antigen. The sections were then incubated with anti-calcitonin antibody at 400C overnight. Slides were then treated with biotinylated secondary antibody, and the reaction was seen using diaminobenzidine as chromagen. All stained sections was assessed qualitatively under the BX-61 computerised microscope and the images were captured with Olympus DP71 camera. The PF cells, stained for anti-calcitonin antibody were observed.
Results
Foetuses collected between 14 to 28 weeks of gestation had CRL ranging between 12.5-26 cm, BPD between 3-6 cm, FL between 1.6-5.1 cm and body weight between 128-524 gms [Table/Fig-1].
Estimated ages and various parameters of foetal specimens.
| S. No | Age inweeks | CRLin cm | BPDin cm | FL in cm | BodyWeight(gm) | NumberCollected |
|---|
| Right | Left |
|---|
| 1 | 14 | 12.5 | 3 | 1.6 | 1.3 | 128 | 1 |
| 2 | 15 | 13.2 | 3.3 | 2 | 2.1 | 172 | 1 |
| 3 | 16 | 15.9 | 4 | 2.4 | 2.2 | 214 | 1 |
| 4 | 18 | 16.8 | 4.2 | 2.6 | 2.6 | 238 | 1 |
| 5 | 20 | 17.5 | 4.4 | 2.8 | 2.5 | 263 | 1 |
| 6 | 23 | 19 | 4.8 | 3 | 3.1 | 351 | 1 |
| 7 | 27 | 24.3 – 24.7 | 5.2 – 5.3 | 4.5 – 4.7 | 4.3 - 4.5 | 498-505 | 2 |
| 8 | 28 | 25.1-26 | 5.8-6 | 5-5.1 | 4.8- 5 | 513-524 | 2 |
(CRL- Crown Rump Length, BPD- Biparietal Diameter, FL- Foot Length)
The histological findings at different gestational ages are:
At 14 weeks: Thin connective tissue capsule surrounding the developing thyroid gland along with blood vessels was present. The parenchyma of the gland at this stage consisted of diffuse network of thyroid cells with very little intervening connective tissue. Smaller blood vessels were seen in the parenchyma. Follicular cells were seen arranged mainly in the primitive form of cords and clusters. At some places, the follicular cells have just started arranging themselves into follicles, on a very thin connective tissue layer of basement membrane. The follicular cells were circular in shape with a darkly stained small round nucleus. The cytoplasm was scanty. Very few pale staining cells with round nucleus with size almost equal to the follicular cells probably PF cells were present, mainly scattered among the follicular cells. They had very little cytoplasm. When present in relation to developing thyroid follicles, they were interfollicular in location in small groups. Immunostaining showed diffuse background staining. Scanty appearance of cells showing expression of calcitonin was seen.
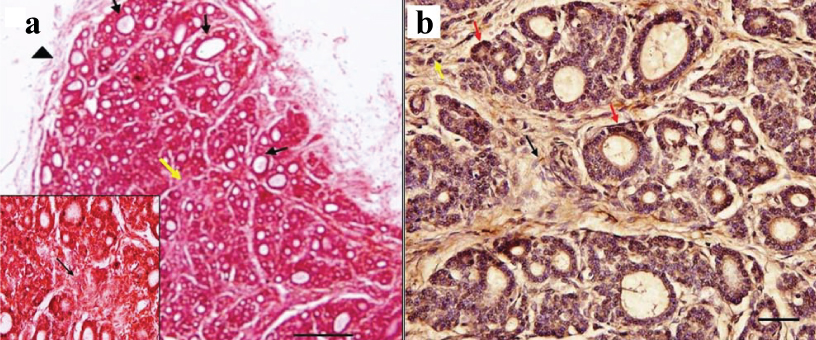
At 15 weeks: The connective tissue capsule surrounding the developing thyroid gland was present which was thicker than the younger foetus containing more fibres, fibroblasts and blood vessels. The capsule was sending septa into the parenchyma. The parenchyma had begun organizing into ill-defined lobules with intervening connective tissue septa. Small blood vessels were seen in the septa. Follicular cells were seen arranged in the form of follicles of various sizes on a thin layer of basement membrane. Larger follicles were located more at the periphery mainly towards the poles especially upper region of the lateral lobe. In some follicles lighter stain colloid was present indicating that the follicular cells have started functioning. In the central region, follicular cells were still present in their primitive form of cords and clusters. More blood vessels had come in relation to the developing thyroid follicles. The follicular epithelium was cuboidal. They had a larger rounded, lighter staining, central nucleus as compared to primitive follicular cells in the younger foetus. The PF cells were not differentiable from follicular cells on H&E. Few solid cell nests were seen in the middle region of lateral lobe. It consisted of central cells with round, pale staining nucleus and peripheral cells with elongated nucleus [Table/Fig-2a]. Immunostaining revealed dark brown coloured calcitonin positive cells which were probably PF cells, in between the bases of the cuboidal follicular cells lining the follicles either singly or in groups. Some immunopositive cells were also seen singly or in groups in interfollicular location. Few cells which were not expressing calcitonin with nuclear morphology similar to PF cells were also seen in interfollicular position. The immunostaining of solid cell nest showed presence cells which were not expressing calcitonin [Table/Fig-2b].
a) H&E staining in 15 weeks foetus, larger follicles (arrows) are located at the periphery in comparison to the center. Thin capsule along with blood vessels is seen surrounding the thyroid tissue. A solid cell nest (yellow arrow) is seen (Scale bar 1 cm=100 μm [10X]). (Inset- magnified view of solid cell nest (black arrow) [40X]); b) Immunostaining with anticalcitonin in 15 weeks foetus, parafollicular cells (red arrows) in intrafollicular location present mostly in groups. Some parafollicular cells (yellow arrow) are seen in interfollicular location too. A solid cell nest (black arrow) is seen, composed of immunonegative cells (Scale bar 1 cm=15 μm [40X]).
At 16 weeks: The general lobular architecture was more organized. The size of lobules has increased. More connective tissue was present between the developing follicles. Large number of follicles of various sizes was seen. In some follicles, slightly darker stain colloid was present than the previous ages. Few primitive follicular cells were scattered within the lobules. The features of follicular epithelium were same as in the previous age. Though in the previous gestational age of 15th week it was difficult to distinguish the PF cells from follicular cells, at the gestational age of 16th week, the PF cells were seen as lighter stained polygonal cells with central, round to oval nucleus with a prominent nucleolus. They were bigger than the PF cells of younger age foetus and the surrounding follicular cells. They were present in the interfollicular location between the developing thyroid follicles in interfollicular connective tissue. They were also present in the intrafollicular location wedged between the follicular cells inside the basement membrane but away from the lumen. At both locations, they were found in groups. The intrafollicular cells were also found singly. Few solid cell nests were also present. Immunostaining showed dark brown coloured calcitonin positive cells probably PF cells were present in intrafollicular location mostly in groups. Some immunopositive cells were also seen in interfollicular location too in small groups.
During 18-20 weeks: Well defined connective tissue capsule was seen. The general architecture was same as in previous age. But the interfollicular connective tissue had decreased. Larger blood vessels were present in the septa. Follicular numbers had increased as compared to younger foetus. Larger follicles were present at the periphery. Colloid was present in some follicles. The primitive follicular arrangement was absent at this age. The features of follicular epithelium was same as in the previous age. But in some follicles stratified epithelium of 5-6 cell layers was noticed. The PF cells were seen in intrafollicular position mostly singly between the darkly stained follicular cells on the basement membrane. They were seen away from the lumen. They were also present in interfollicular location in small groups of 2-3. It was seen as pale staining round to oval nucleus which was larger than the follicular cells. Prominent nucleolus was present. Immunostaining showed black to dark brown immunopositive cells probably PF cells mainly intrafollicular in location. PF cells were also present in groups in interfollicular location. The colloid was showing light immunopositivity (light brown). Blood vessels also showed light immunopositivity.
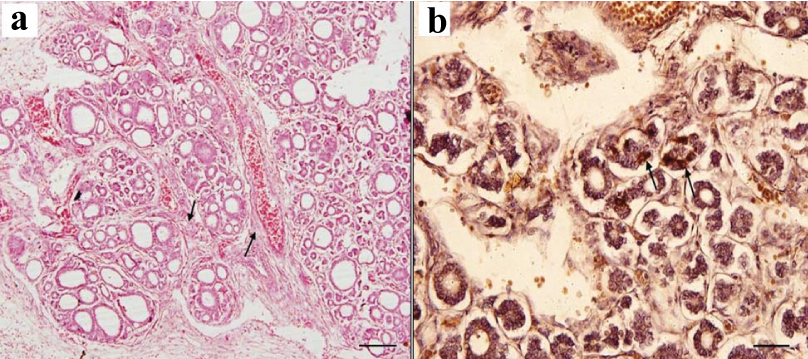
At 23 weeks: The features of parenchyma was same as in the previous age. However, due to growth and proliferation, the size of the lobes appeared larger. The follicles have increased in size. Large size follicles were seen in the centre. Colloid was present in some follicles. The features of follicular epithelium was same as in the previous age. They were present mostly in intrafollicular location and also in groups in interfollicular position. PF cells were seen as pale staining polygonal cells with round to oval nucleus which were larger than the follicular cells [Table/Fig-3a]. On immunostaining, dark brown immunopositive cells probably PF cells, mainly intrafollicular in location were seen. Many immunopositive cells in interfollicular location were also present in small groups. The blood vessels also showed immunopositivity [Table/Fig-3b].
a) H&E staining in 23 weeks foetus, thick septa (black arrows) dividing the thyroid tissue into many small lobules. (Scale bar 1 cm=100 μm [10X]); b) Immunostaining with anticalcitonin in 23 weeks foetus, showing dark brown immunopositive parafollicular cells which are intrafollicular (black arrows) in location. Immunopositivity is also seen in blood vessels (Scale bar 1 cm=15 μm [40X]).
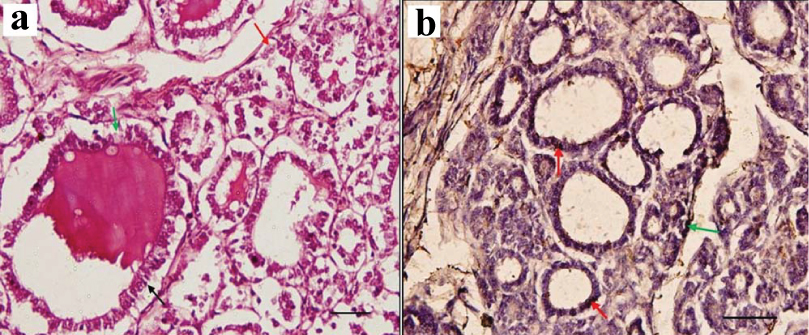
At 28 weeks: The architecture of the lobes and lobules was highly developed in the parenchyma. The intralobular connective tissue was minimal. Follicles of almost equal sizes are now seen all throughout the parenchyma. Colloid was present in some follicles. Vacuoles are present in the colloid. Follicular epithelium was cuboidal to columnar. They had darker stained rounded nucleus [Table/Fig-4a]. No primitive follicular arrangement was seen. The PF cells were seen as lighter staining polygonal cells with round to oval nucleus which was larger than the follicular cells. Prominent nucleolus was present. It was present in intrafollicular position mostly singly between the darkly stained follicular cells on the basement membrane. It was seen away from the lumen. They were also present in interfollicular location in small groups. Immunostaining showed many dark brown coloured calcitonin positive cells probably PF cells mostly in intrafollicular location in groups. Few immunopositive cells were also seen in interfollicular location too in small groups. These interfollicular cells were mostly located near the blood vessels. Rest of the cells in the follicular epithelium which had stain bluish due to counterstaining with hematoxylin were probably follicular cells. The colloid was not expressing calcitonin [Table/Fig-4b].
a) H&E staining in 28 weeks foetus, showing follicles lined by columnar cells (black arrow), dark stained colloid and vesicles in the colloid. Parafollicular cells are seen as cells with lightly stained nucleus, both, singly in intrafollicular location (green arrow) and in groups of two to three in interfollicular location (red arrow) (Scale bar 1 cm=15 μm [40X]); b) Immunostaining with anticalcitonin in 28 weeks foetus, showing immunopositive parafollicular cells. Red arrow indicates intrafollicular location and green arrow indicates interfollicular location of parafollicular cells near a blood vessel. Immunopositivity is mainly seen in intrafollicular location (scale bar 1 cm=15 μm [40X]).
Discussion
The study of PF cells development is essential for understanding its effect on developing thyroid follicles and its role in the foetal development process. The mammalian PF cells are derived from the neural crest cells which get incorporated into the ultimobranchial body. These ultimobranchial bodies get incorporated into the thyroid primordium to give rise to the PF cells [5,6] and its remnants, the solid cell nests and the mixed follicles. About 9.7%- 30% of adult thyroid gland has these remnants of solid cell nests [7,8]. Sonic Hedgehog (Shh) has been proposed as a controller of migration of PF cell precursors into the thyroid primordium [9]. Lately, lineage tracing in Sox17 labelled mice has concluded that the mouse PF cells are derived from pharyngeal endoderm of developing foregut [9].
The youngest foetus in our study belonged to the 14th week of gestation. Thus, the ultimobranchial body had already incorporated into the developing thyroid. At this stage, the PF cells were very few and scattered among the follicular cells. Only in few developing thyroid follicles, they were interfollicular in location in small groups. This is in contrast to study by Chan AS et al., [10] in which they reported both intrafollicular and interfollicular locations of PF cells. The genes involved in the distribution of PF cells throughout the thyroid parenchyma are Nkx2.1, Eya1, FRS2α and Hox3 paralogs [9]. The immunostaining showed diffuse background staining with scanty calcitonin positive stain cells. It is in accordance to the literature stating that the immunoreactive calcitonin containing cells were constantly observed from 14 weeks of gestation before the appearance of follicular structures [11]. It is also important to note that although the PF cells had been shown to be present within the thyroid gland as early as 9 weeks of gestation, they remain poorly differentiated until 14th week [10,11]. As we did not have younger foetuses, we could not find out if these PF cells were present earlier than 14 weeks. In the present study, the PF cells were not easily identified by their morphological characteristics, but there was a number of undifferentiated cells showing expression of calcitonin antibody. Thereby, we can infer that though the PF cells are not morphologically identifiable, they are functional. This is in concurrence with the literature which refers that the plasma calcitonin level at 14 weeks is appreciable [12]. Also, the diffuse staining might indicate the increased production of calcitonin hormone at this stage which had dispersed into the surrounding connective tissue. This high level of calcitonin in the thyroid gland indicates that it has an important role in maintaining calcium levels at this stage of gestational period. In the literature, it is mentioned that it might have a role in ossification or maintaining magnesium homeostasis in human foetus [13]. The maturation of follicles has been divided into three periods- precolloid (7-13 weeks), colloid formation stage (13-14 weeks), follicular stage (14 weeks onwards). More recently, a fourth stage of secretory activity (20th-24th week) has also been suggested [14]. At this stage in our study, the developing thyroid gland was in the precolloid phase.
In the 15 weeks old foetus, the PF cells were present both in interfollicular location in groups of variable numbers and also in intrafollicular location either singly or in small groups. So, at this stage, the PF cells had started organizing itself from interfollicular to intrafollicular location. This change in orientation of PF cells is due to the presence of desmosomes between PF and the follicular cells [15]. In immunostaining, the surrounding interfollicular connective tissue was taking up the stain but to a lesser degree than the previous age group indicating more localization of PF cells. Gaikwad JI et al [14] stated that the differentiation of epithelial cells into follicles starts at the periphery around 12 weeks and extends centrally. In the present study, larger follicles were located more at the periphery as compared to the central region where still some follicular cells was present in their primitive form of cords and clusters. In some follicles, lighter stain colloid were present indicating that the follicular cells have started functioning at this stage of gestation. A small number of solid cell nests were present which was not expressing calcitonin suggesting that they might be undifferentiated cells. The literature states that they are the remnants of the ultimobranchial bodies consisting of main cells which are polygonal to elongated cells with a central nucleus and C-cells which had a compact nucleus [8].
At 16th week of gestation, Leroyer AE et al., [11] reported scattered PF cells in the developing thyroid. To this contrary in our study, they were present almost equally in the interfollicular location as well as in intrafollicular location in groups. The intrafollicular located cells were also found singly. The PF cells appeared morphologically matured as adult PF cells. The genes involved in the differentiation PF cells are Hes1, Mash1 and Nkx2.1 [9].
In the 18 to 20 weeks’ foetuses, the PF cells were present mostly in the intrafollicular position. This shows that the PF cells are getting more and more embedded into the follicles from initial interfollicular location. The surrounding connective tissue is also getting lighter immunostain due to organization of PF cells. These findings were in accordance to the study in foetal thyroid that states; at 165 mm crown rump length (ff. 18 weeks) foetus, the thyroid was fully differentiated and definitive follicles were present in which the PF cells were present singly or in small groups in both intrafollicular and parafollicular location [10]. Absence of primitive follicular arrangement at this gestation suggests that the developing thyroid has completed the follicular stage of development.
In the 23 week foetus, the PF cells were present mostly in intrafollicular location with few cells in interfollicular position. This supports the literature that by 20th to 27th week most of the PF cells are intrafollicular [11].
In the 28 week foetus, the PF cells were also seen mostly in intrafollicular position. This backs the finding in literatures that in neonates PF cells are mostly intrafollicular in location [11,16]. Few solid cell nests were also seen indicating that some undifferentiated cells still exit at this stage. Follicles of almost equal sizes are now seen all throughout the parenchyma. Signs of increased secretory activity of the follicular cells indicate the start of the secretory phase of follicular development.
Limitation
A quantitative assessment of sequential increase/ change in number of follicular and parafollicular cells using stereology techniques was not done as facility for stereology was not available. This study could be further elaborated by use of other functional and maturation specific immunostain for parafollicular cells.
Conclusion
In our study, the PF cells were seen as earlier as 14th week. They became functionally and morphologically mature by 16th week of gestation. The sequential development of PF cells from a very primitive form to a more organised form along with the follicular development was traced. These findings could be further correlated with electron microscopic study of developing PF cells to find the cause of such organization.
The decrease in the intensity of immunostain of the thyroid parenchyma with gestational age points towards the higher role of calcitonin in early gestational age. As calcitonin inhibits osteoclastic activity and increase osteoblasts numbers, it supports the hypothesis that it might play a contributing factor in the regulation of ossification in the human foetus.
Declaration
This paper was presented in 62nd National Conference of Anatomical Society of India at Imphal in 2014.
(CRL- Crown Rump Length, BPD- Biparietal Diameter, FL- Foot Length)