Spinal anaesthesia is the most popularly performed procedure in the field of anaesthesiology. Hyperbaric bupivacaine, the local anaethetic most commonly used, has limitation as its effect lasts only for 1.5-2.0 hours [1-3]. Hence a lot of adjuvants have been tried to enhance the analgesic effect of bupivacaine. Opioids have been found to prolong anaesthesia and analgesia, have been seen to improve the quality of analgesia and provide haemodynamic stability [4,5]. Opioid and local anaesthetic eliminate pain by acting at two different sites. Local anaesthetics act at axon level and opioids act on the receptors present on spine.
Nalbuphine is a member of the opioid family. It is an antagonist of μ receptor but agonist of kappa receptors [6,7]. It was synthesized in an attempt to produce analgesia without the undesirable side effect of alpha 1 agonist. Nalbuphine in a dose of 0.4 mg, when added intrathecally, improved the quality of intraoperative and postoperative analgesia and had a fewer side effects as compared to 0.4 mg of morphine [8-10]. Respiratory depression and abuse potential with nalbuphine is very less on comparing with other centrally acting opioid.
In our institution, fentanyl is commonly used as spinal adjuvant. On comparing nalbuphine with fentanyl, the later is costlier and needs narcotic licensing. We have compared the analgesic efficacy of nalbuphine with fentanyl as well as the associated adverse effects. Few studies have investigated intrathecal nalbuphine with hyperbaric bupivacaine [10,11] and as far as we know, only one study has compared it with intrathecal fentanyl in patients undergoing caesarean section [12]. Hence, we have compared whether fentanyl or nalbuphine is a better additive for bupivacaine and if it is nalbuphine then what dose of nalbuphine (0.8 mg or 1.6 mg) is better.
Materials and Methods
After approval of the Institutional Ethical Committee and obtaining written informed consent from the patients, a double blinded randomised clinical study was conducted on 90 adult patients of ASA I and II, posted for lower limb orthopaedic surgeries under spinal anaesthesia. This study was conducted between December 2015 to August 2016.
Inclusion criteria were; age between 18-60 years, either sex, height between 160±10 cm, weight between 50-75 kg, patients with ASA I and II status and undergoing lower limb orthopaedic surgery in spinal anaesthesia which were expected to take time of 120-150 minute. Exclusion criteria were; patient on beta blocker therapy, ASA III and IV status, any contraindication to central neuraxial block, patient with known hypersensitivity to any of the study drugs, pregnancy and patient on sedatives and tranquilizers. The sample size was calculated by Balance ANOVA method which was 90. Patients were randomly allocated into three groups using computer generated random number, each group consisting of n=30. Each patient received tab alprazolam 0.25 mg and tab pantoprazole 40 mg the night before surgery as premedication. Peripheral intravenous cannulation and preloading was done in the operation theatre. Patients were attached to multichannel monitors and routine monitoring like non invasive blood pressure, pulse oximetry, electrocardiogram were done. Group F received 25 μg of fentanyl with 12.5 mg of 0.5% hyperbaric bupivacaine, Group NL received 0.8 mg of Nalbuphine with 12.5 mg of 0.5% hyperbaric bupivacaine and Group NH received 1.6 mg of nalbuphine with 12.5 mg of 0.5% hyperbaric bupivacaine. Syringe preparation and drug administration was done by an independent anaesthesiologist (not involved in the study).
Patients were placed on operation table in sitting position. Under aseptic condition, by midline approach, 25 G sterile disposable spinal needle was introduced at L3-4 intervertebral space and drugs were administered slowly according to the group of the patient.
The sensory and motor level of the subarachnoid block in the patient was monitored at different time intervals. After operation patients were monitored for 24 hours in the postoperative care unit and necessary recordings were done.
Respiratory depression (RR<8 or SpO2 <95%) could be treated with oxygen supplementation or respiratory support if required. Hypotension defined as a decrease of systolic blood pressure by > 30% from baseline or a fall below 90 mmHg, was treated with incremental Intravenous (IV) doses of 6 mg of injection mephentermine and IV fluid as required. Bradycardia i.e., Heart Rate (HR) below 50 bpm, was treated with 0.3-0.6 mg of IV atropine. HR, Mean Arterial blood Pressure (MAP) and oxygen saturation (SpO2) were monitored and recorded after the block every five minutes till 30 minutes, then at 15 minute interval upto 120 minute and at 30 minute interval thereafter. Adverse effects such as nausea, vomiting, shivering, respiratory depression, sedation and hypotension were recorded. Sensory testing was assessed by loss of pinprick sensation to 23 G hypodermic needle. Dermatome levels were tested every two minute until the highest level was stabilised by consecutive testing. Surgery was allowed on achieving T8 level of sensory blockade. Then testing was conducted every 10 minutes until the point of 2 segment regression of the block was observed. Further testing was done at 20 minutes interval until the recovery of S1 dermatome. Data were recorded regarding highest dermatome level of sensory blockade, the time to reach this level from the time of injection, time to S1 level of sensory regression, time to urination and incidence of side effects. The motor block assessment was done using the modified Bromage Scale [13]. Pain assessment was done using a 10 point Verbal Rating Scale (VRS) varying from 0 to 10 (0 meaning no pain, 5 for moderate pain and 10 for worst imaginable pain). After spinal injection pain was recorded every 30 minute for two hours, then at every 15 minute till first four hours or till the patient complained of pain, whichever was later, followed by two hourly assessment till the eighth hour and four hourly till 24 hours. For effective comparison and to avoid indivualized analgesic needs we reocorded highest VRS score achieved and Cumulative Analgesic Consumption Score [14] (CACS). CACS was recorded using VRS scale till 24 hours. When VRS score was 1-3 CACS was 1, VRS score 4-6 CACS 2 was given and when VRS score was 7-10 then CACS of 3 was given.
Ramsay sedation scale [15] was used to assess sedation before the block and after every 15 minutes. HR, MAP, oxygen saturation (SpO2) and sedation score were recorded postoperatively, initially every one hour for two hours, then every two hour for next eight hours and then every four hour till 24 hours. The duration of pain relief was defined as the time from spinal injection to the first request for rescue analgesics. The attending anaesthesiologist was advised to give rescue analgesia on demand with intramuscular diclofenac, initial dose of 75 mg followed by 100 mg intravenous tramadol as needed. Analgesic requirement for the first 24 hours was on demand “only”. Maximum allowable dose of Diclofenac was 150 mg/day and for tramadol it was 400 mg/day. Total dose of analgesic required in 24 hours was recorded.
Statistical Analysis
The statistical analysis was done using Statistical Package for Social Science evaluation (SPSS) version 16.0. Results were expressed as mean, standard deviation and range. Frequencies were expressed as number and percentage. One-way analysis of variance (ANOVA) was used for multiple group comparison and intergroup data analysed by students t-test (numerical) and chi-square test (categorical). A p-value of 0.05 or less was considered significant for statistical analysis.
Results
Ninety patients were enrolled. There were no significant differences in demographic data regarding age, weight, height, sex, ASA grading and duration of surgery, among the different groups i.e., F, NL and NH as shown in [Table/Fig-1].
| Characteristics | Group F | Group NL | Group NH | p-value* |
|---|
| Age (years) | 39.1±12.34 | 41.4±15.97 | 39.7±16.01 | 0.9379 |
| Sex (M:F) | 6:4 | 6:4 | 7:3 | |
| Weight (kg) | 57.4±8.08 | 58.1±8.49 | 60.5±12.39 | 0.7636 |
| Height (cm) | 161.75±9.13 | 162.5±8.16 | 166.25±16.01 | 0.5163 |
| ASA I:II | 8:2 | 7:3 | 8:2 | |
| Duration of Surgery (minutes) | 120±37.93 | 130.5±33.20 | 120±20.57 | 0.7037 |
*Test applied: ANOVA
The results regarding the characteristics of sensory and motor blocks have been shown in [Table/Fig-2]. The time of onset of sensory and motor blocks were insignificantly less in F group than NL which was insignificantly less than NH. Time of two segment sensory regression were comparable between groups NL and NH and were significantly less (p=0.03) when compared with F group. Median level of peak sensory block was T6 in all the three groups. The duration of sensory and motor blocks were comparable in all the three groups.
Characteristics of sensory and motor block.
| Variables | Group F | Group NL | Group NH | ANOVAp-value |
|---|
| Time to reach max sensory level (min) | 8.1±3.84 | 9.8±4.15 | 12.3±6.73 | 0.196 |
| Peak sensory level | T6 (T6-T10) | T6(T4-T8) | T6(T4-T10) | |
| Time of two segment sensory regression | 108±32.03 | 91.6±31..12 | 73.5±17.95 | 0.038 |
| Time to reach complete motor block | 5.4±12.96 | 7.4±3.13 | 10.4±4.5 | 0.433 |
| Duration of motor blockade (min) (regression to bromage 0) | 204.5±40.03 | 177.5±50.45 | 202.5±65.28 | 0.456 |
| Time of sensory regression to S1 level | 232±61.96 | 248±40.22 | 222±58.65 | 0.566 |
*Test applied: ANOVA
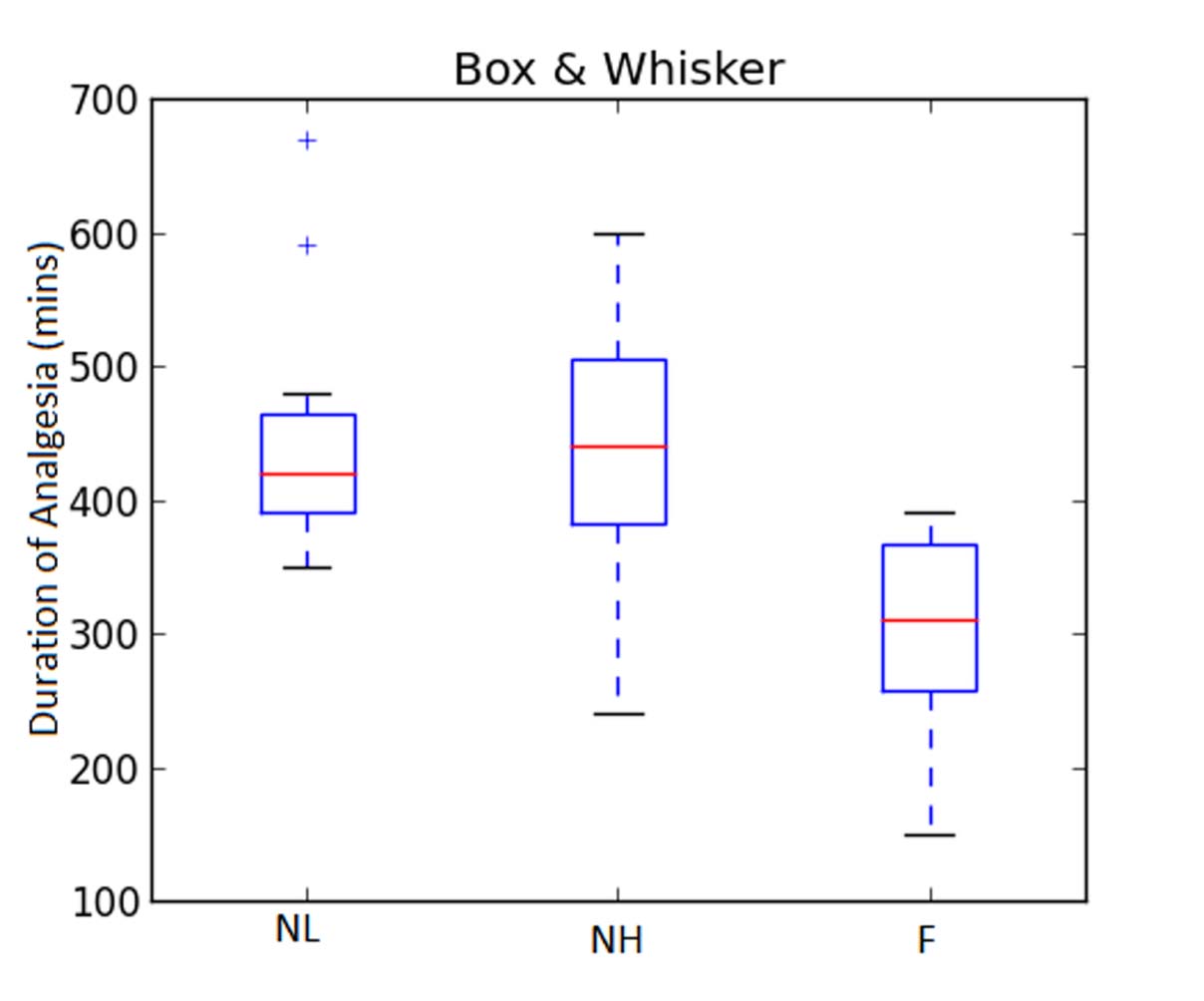
The duration of analgesia (in minutes) was 441±119.69 in NL Group, 450±109.38 in NH Group and 300.0±88.53 in Group F (p=0.005). There was significant difference in the duration of analgesia between Group NL and F [Table/Fig-3,4].
Effects of study drug on pain and sedation.
| Variables | NL Group | NH Group | F Group | p-value* |
|---|
| Duration of Analgesia (Mins) | 441±119.69 | 450±109.38 | 300±88.53 | 0.0051 |
| Highest VRS Score | 3±1.4 | 3.9±1.7 | 5.5±1.8 | 0.009 |
| CACS** | 1.875±0.83 | 2.25±0.7 | 3.375±1.77 | 0.0186 |
| Sedation Score | 1.30±0.39 | 1.76±0.52 | 0.46±0.60 | 0.026 |
*Test applied ANOVA ** Cumulative analgesics consumption score
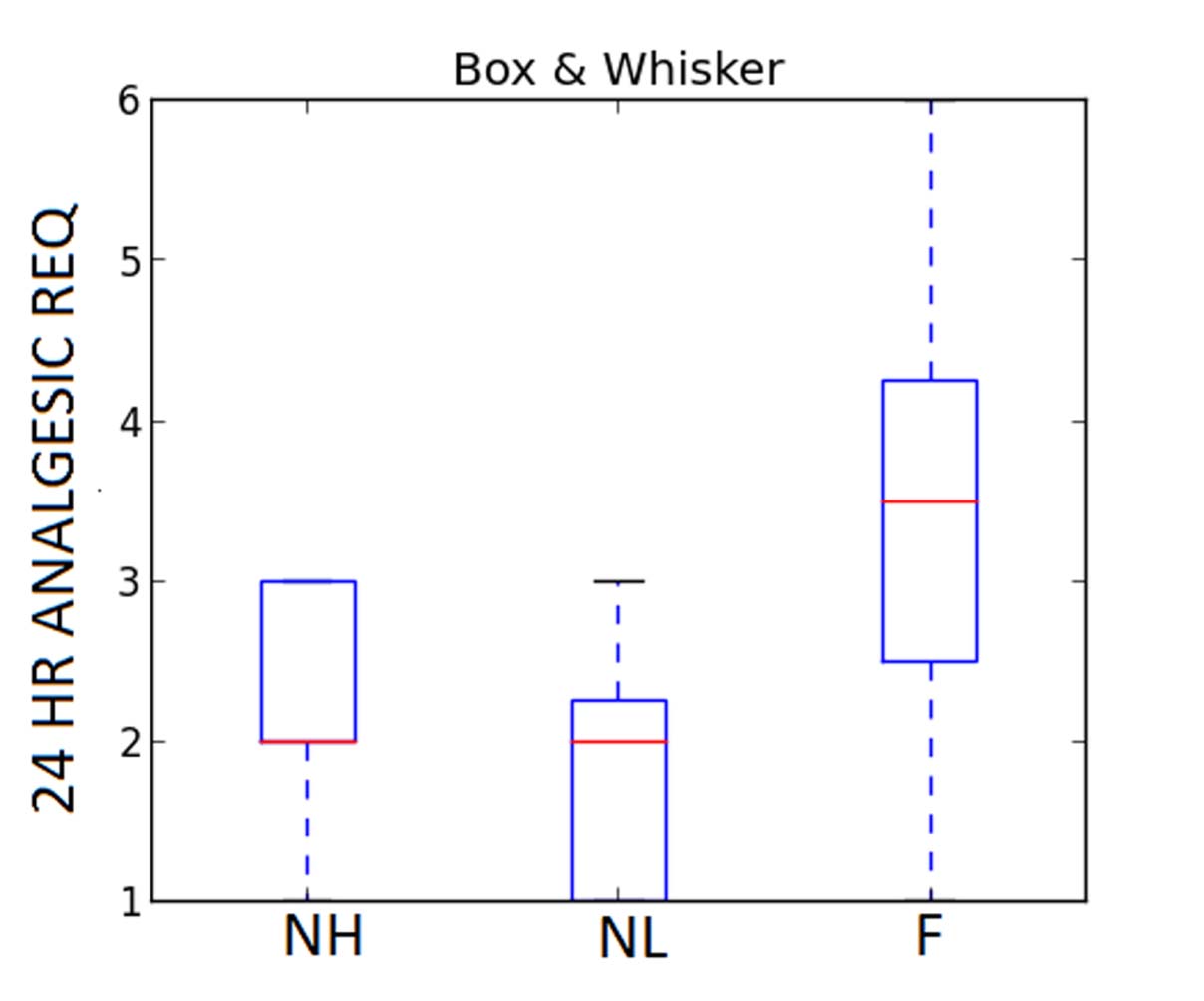
Total 24 hours analgesic requirement was titrated by analgesic score which was 2.25±0.7 (NH Group), 1.875±0.83 (NL Group) and 3.375±1.77 (F Group) p=0.0186 by ANOVA. Hence, the requirement of 24 hour rescue analgesics in terms of total number of doses were significantly less in Group NL when compared to Group F [Table/Fig-3,5].
Total 24 hour analgesic requirement.
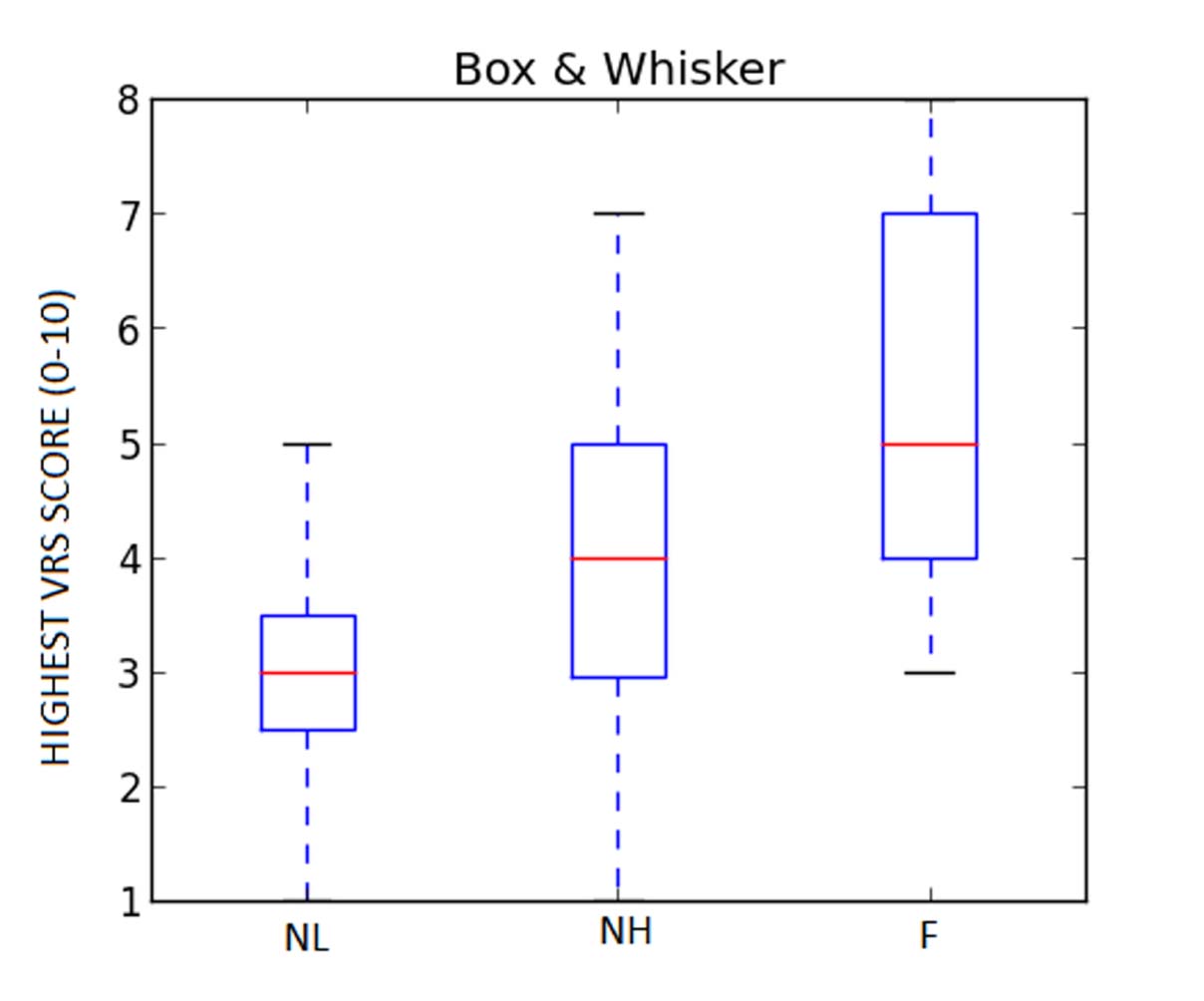
The mean of highest VRS score during 24 hour time were NL (3±1.4), NH (3.9±1.7) and (5.5±1.8) groups. Thus, there was adequate postoperative analgesia in all the groups. VRS score was least in NL Group and highest in F Group and the difference was statistically significant (p=0.009) [Table/Fig-3,6].
Mean of highest VRS score.
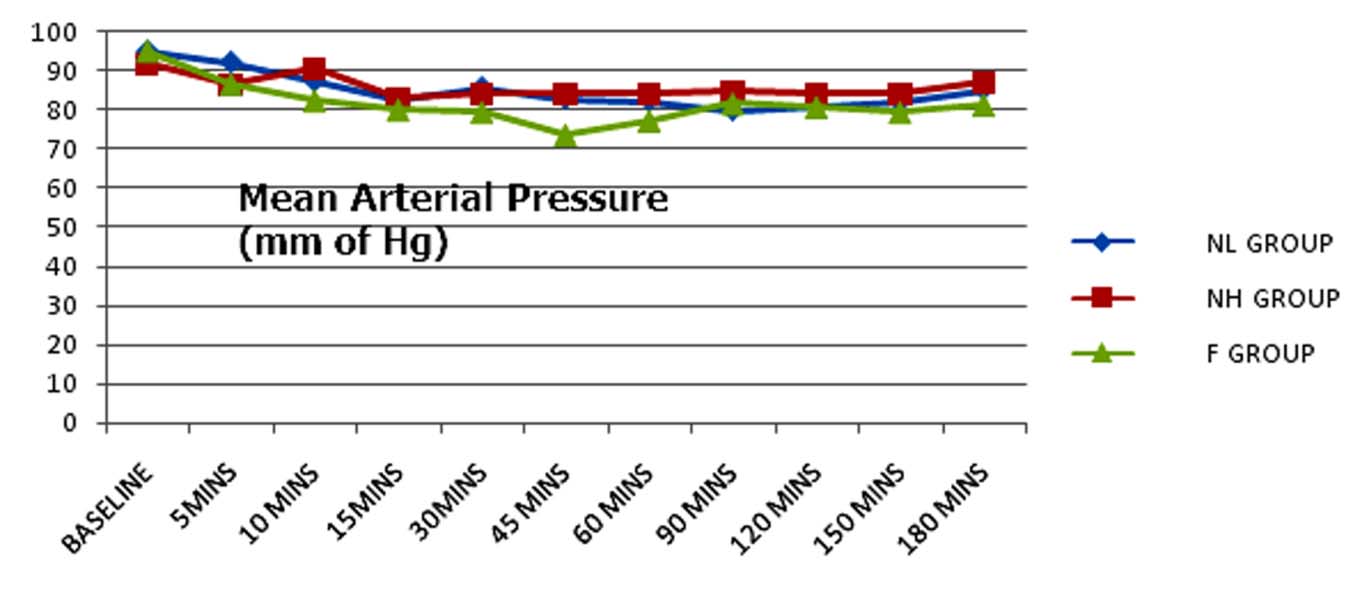
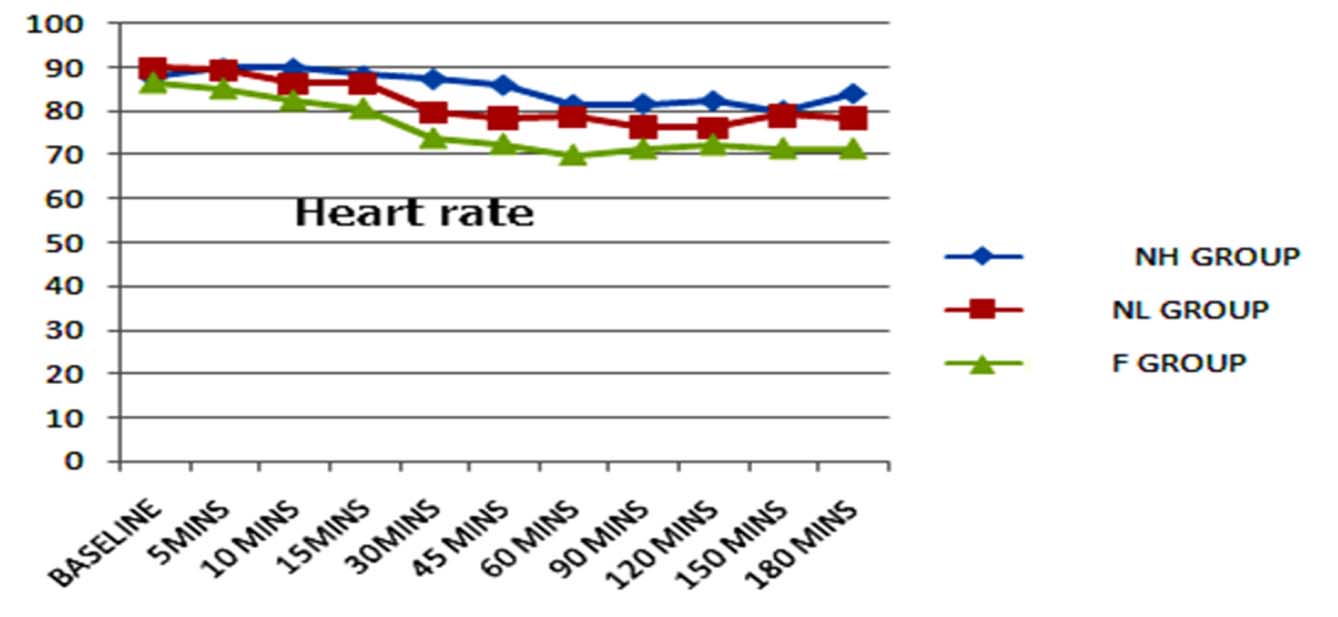
All the three groups were haemodynamically stable [Table/Fig-7,8]. Six patients in Group F, three patients in NL Group and none of the patients in NH Group reported hypotension which was mild and easily corrected by giving one or two boluses of injection mephentermine.
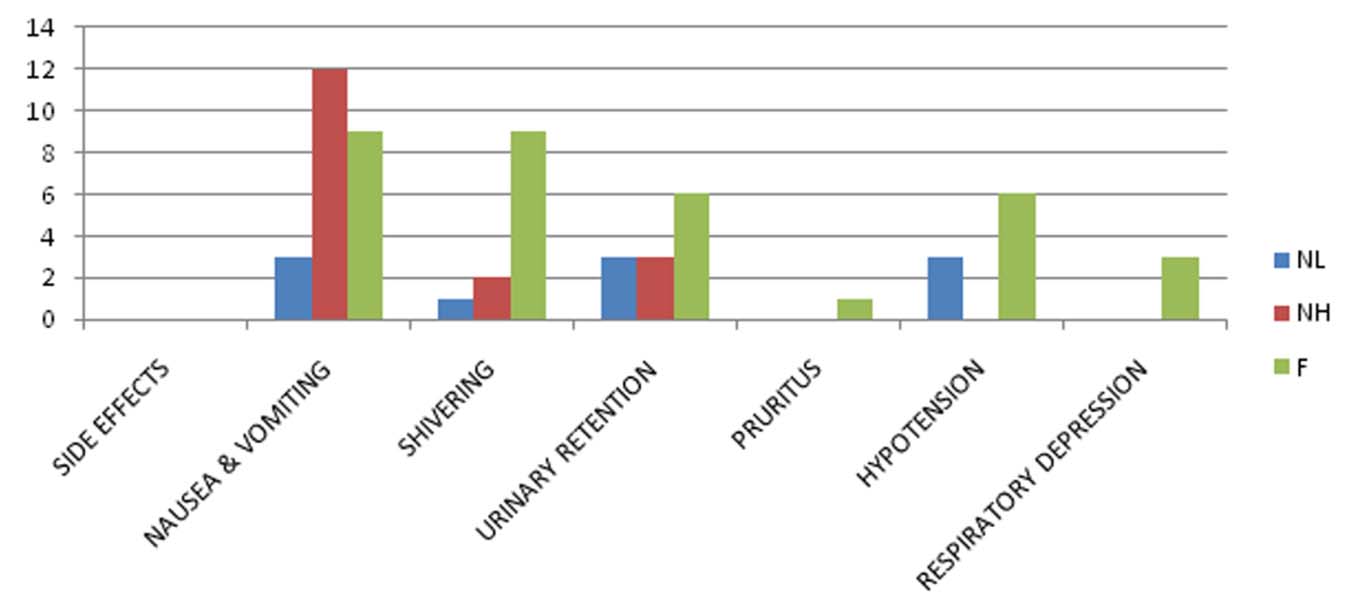
Side effects among the groups have been summarised in [Table/Fig-9]. The adverse effects were more in fentanyl group than nalbuphine groups. These were comparable between NH and NL groups. The incidence of nausea and vomiting was maximum in NH followed by F than NL.
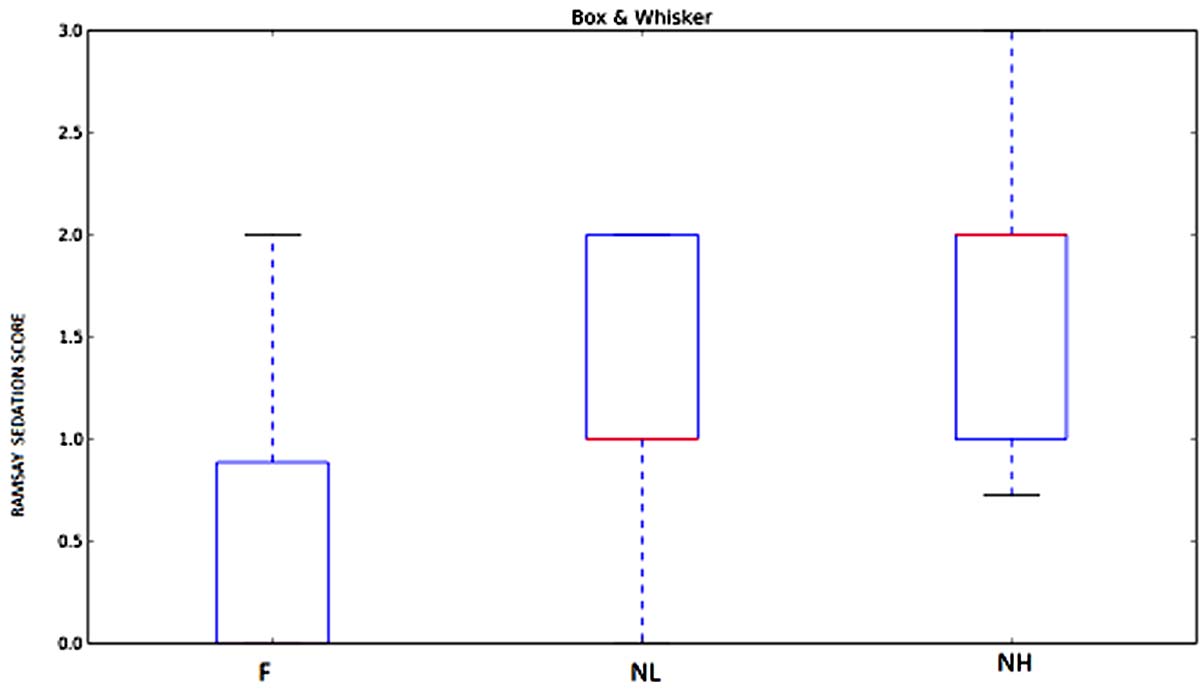
Mean sedation score was 1.30±0.39 in NL Group, 1.76±0.52 in NH Group and 0.46±0.60 in F Group [Table/Fig-3,10]. Patients in nalbuphine-bupivacaine group were sedated but easily arousable and there was no respiratory depression in any of them. So sedation was advantageous as the patients were calm and did not require added sedation.
Discussion
Intrathecal opioids are quite commonly used as adjunct to local anaesthetics in regional anaesthesia with multiple advantages. The most common causes of mortality in regional anaesthesia are high spinal and local anaesthetic toxicity. Hence, reduction in the doses of local anaesthetics and better management of local anaesthetic toxicity is possible in this way [16]. Opioids intrathecally decrease nociceptive inputs form A delta and C fibres without affecting dorsal root axons or somatosensory evoked potentials [17]. Nalbuphine when binds to μ receptors, competitively displaces other μ antagonists from the receptors without itself displaying any agonistic effect. When it binds to kappa receptors, it has agonistic effect. Hence, it is a mixed agonist-antagonist. It produces analgesia and sedation without μ side effects. Animal studies have ruled out any neurotoxicity of intrathecal nalbuphine [18].
In this study, the postoperative analgesic requirements and spinally mediated analgesic effects of 0.5% hyperbaric bupivacaine in combination with either fentanyl or two doses of nalbuphine were studied and recorded. Onset and duration of sensory block were faster in fentanyl than nalbuphine groups, though statistically insignificant. It may be due to high lipid solubility and rapid tissue uptake of fentanyl. In our study, there was no statistically significant difference in the duration of sensory or motor blocks between the groups and their haemodynamics. This finding is similar to HM Gomaa HM et al., [12]. There was no bradycardia in any of the study groups. Fentanyl being a μ agonist, respiratory depression was seen in three of the patients in this group and none of the patients of NL and NH Group reported this. The duration of post operative analgesia was maximum in NL followed by NH which followed F group, with significant difference statistically [Table/Fig-5]. The side effects were more in F and comparable in nalbuphine groups. None of the patients of NH Group showed any inidence of hypotension or bradycardia but PONV was maximum in this group. If we consider the 24 hour analgesic consumption, it was maximum in Group F and least in NL Group and there was significant difference between the two groups. VRS score during 24 hour period, was more in F and least in NL group. This also differed significantly. Culebras X et al., who compared intrathecal morphine with intrathecal nalbuphine in different doses viz., 0.2 mg, 0.8 mg and 1.6 mg concluded that intrathecal nalbuphine 0.8 mg provides good intraoperative and early postoperative analgesia, without side effects [10]. They found that intrathecal nalbuphine 1.6 mg did not increase the analgesic efficacy but the side effects increased in this group. Hence they recommended the dose of 0.8 mg for intrathecal injection after caesarean section. Jyothi B et al., also observed that increasing Nalbuphine dose from 0.8 to 1.6 mg and 2.4 mg did not increase analgesic efficacy [19]. That means nalbuphine exhibits a ceiling effect to analgesia i.e., increase in the dose of drug increases analgesic effect only upto a certain point beyond which there is no further increase in this effect with the increase in dose. Our result also correlates with Culebras X et al., and Jyothi B et al., in this respect [10,19]. Gomaa HM et al., compared postoperative analgesia between 25 μg of intrathecal fentanyl with 0.8 mg of nalbuphine and did not find any significant difference in the duration of analgesia between the two [12]. But we found significant difference between the same. They did not study 24 hours analgesic consumption and VRS score in 24 hours. Studies done by Tiwari AK et al., Mostafa MG et al., reported that nalbuphine prolonged duration of analgesia with reduced VAS pain score [11,20].
Patients who received nalbuphine-bupivacaine combinations were sedated, calm, and easily arousable with verbal commands. Studies conducted by Culebras X et al., Tiwari AK et al., Mostafa MG et al., showed similar results [10,11,20]. Fewer patients of F Group had sedation and that too of Grade 1 score.
This study has been done in the age group of 18-20 years. Studies in extremes of age is needed to be done to see the effect of the study drugs. Also, obese and low weight subjects have not been taken into considerations. The effect of the study drugs in people with other comorbidities like diabetes and hypertension is not known and needs further studies.
Conclusion
Nalbuphine hydrochloride Groups NL (0.8 mg), NH (1.6 mg) and fentanyl group (F) prolong duration of sensory blockade, provides very good quality, and longer duration of postoperative analgesia. There is no significant advantage of intrathecal fentanyl or 1.6 mg nalbuphine over low dose 0.8 mg nalbuphine. We conclude that NL Group is the best among the three groups.
*Test applied: ANOVA
*Test applied: ANOVA
*Test applied ANOVA ** Cumulative analgesics consumption score