Type 2 Diabetes Mellitus (T2DM) is characterized by progressive loss of beta cells of pancreas leading to failure of anti hyperglycaemia therapy. With increasing duration of T2DM, insulin therapy may become essential in majority of the patients [1]. Estimates from the year 2015 suggest that there are 69.2 million diabetic patients in India and this number is projected to increase to 123.5 million by the year 2040 [2]. T2DM is the major form of DM in Indians accounting for 90% of total diabetes burden. Besides being significant in prevalence, diabetes also has significant socioeconomic impact [3]. Reducing hyperglycaemic burden with achievement of glycaemic targets is known to reduce microvascular and macrovascular complications of diabetes [4,5]. Thus, the control of glycaemia is essential to reduce disease related and social complications arising due to T2DM.
Insulin is the most potent hypoglycaemic agent that has undoubted place in the management of uncontrolled T2DM [1]. As an add-on to the OAAs, basal insulin is the recommended as initial insulin [6]. In Indian patients with T2DM, insulin therapy has been found effective in improving glycaemic control and quality of life [7]. Insulin glargine, one of the initial basal insulins, administered as once-a-day therapy is found to have equivalent efficacy as compared to the repeated short-acting insulin with advantages of low hypoglycaemia, better patient satisfaction and fewer injections [8]. Also, basal insulin therapy given for six months duration has been reported to provide better long term glycaemia control compared to OAAs [9]. Insulin glargine is associated with lower hypoglycaemia risks than other insulin regimens [10]. Given these advantages with basal insulin glargine, we studied its efficacy and safety in patients with T2DM, uncontrolled on single or two OAAs.
Materials and Methods
The present prospective, open label, observational study was conducted at a Diabetes unit of the Internal Medicine Department of a Tertiary Care Hospital in New Delhi, India. Study duration was two years i.e., between December 2012 to December 2014. Study protocol was approved by Institutional Ethical Committee. Informed consent was obtained from all patients before entry in to the study. Patients of uncontrolled T2DM were recruited. Inclusion criteria were adult patients aged above 40 years, inadequate glycaemic control with monotherapy or two drug combination therapy for three consecutive months as suggested by HbA1c of 8% and above on background of dietary and lifestyle measures, FBG above 140 mg/dl. Excluded were those patients with triple oral drug therapy, pregnant women, those receiving steroid, known history of allergic reaction to insulin, renal dysfunction (serum creatinine >1.5 mg/dl]. One week run-in period was allowed for the patients to understand the study, follow the instructions clearly and to assess their adherence to the study. All patients were advised similar level of dietary and lifestyle restrictions.
Patients were started with 10 units of insulin glargine at bed time as an add-on to the current oral hypoglycaemic treatment. In this 12-week study, each patient was followed weekly for insulin dose titration based on FBG and PPBG. At baseline and at each visit, weight, blood pressure, FBG and PPBG was recorded. HbA1c (%) was evaluated at baseline and at the end of 12-week period. All the biochemical investigations were carried out at hospital central laboratory. Blood glucose estimation was performed with glucose oxidase-peroxidase method. Glycosylated haemoglobin was estimated by cation exchange resin method using glycol-haemoglobin reagent set. As patients were initiated for the first time with insulin treatment, they were followed weekly to detect early changes in glycaemia and to detect incidence of hypoglycaemia. At each visit, detailed clinical examination, assessment of patient diaries for hypoglycaemia episodes, and blood sugar (FBG and PPBG) were performed. Patients unwilling to follow up weekly were excluded. All patients were reminded telephonically to maintain adherence to study visits. Patients who did not return for follow up visit even after telephonic reminders within two days of scheduled visits were excluded from the study.
Patients were asked to maintain a diary to record any events related to therapy. Patients were educated on symptoms of hypoglycaemia at baseline and were asked to record such symptoms with their relation to time and dosing of insulin in patient diaries. For each patient, adherence to continued lifestyle modification was ensured at each visit.
Efficacy was assessed by glycaemic changes from baseline to 12 weeks in HbA1c, FBG and PPBG and achievement of goal HbA1c of <7%. Incident hypoglycaemic events were considered in safety assessments. Symptomatic hypoglycaemia was defined as observed or felt symptoms of hypoglycaemia as recorded in diaries treated with self ingestion of sugars followed by recovery from symptoms or glucometer reading of < 50 mg/dl. Severe hypoglycaemia was defined as requirement of admission and intervention in hospital. Episodes of hypoglycaemia after taking bed time insulin that occurred between 10 pm to 7 am were regarded as nocturnal hypoglycaemia.
Statistical Analysis
Statistical analysis was performed by the SPSS program for Windows, version 17.0. Continuous variables are presented as mean ± SD, and categorical variables were presented as absolute numbers and percentage. Data were checked for normality with Shapiro-Wilk normality test. Normally distributed continuous variables were compared from baseline to 12-week using paired t-test. Categorical variables were analysed using either the chi-square test or Fisher’s exact test. A p-value of <0.05 was considered as statistically significant.
Results
Baseline Characteristics
Out of 46 patients recruited, 40 patients were followed till 12 weeks. Six patients were excluded; four patients did not follow up weekly and two were shifted to split mix regimen of insulin for diabetes control. Mean age was 56.35 years with slightly higher proportion of patients having age above 50 years (55.0%). Percentage of females was slightly more than males. Mean age of patients at diagnosis of diabetes was 44.7 years and mean duration of diabetes was 11.6 years. Baseline characteristics are summarized in [Table/Fig-1].
Demographic characteristics of study patients.
| Characteristics | Observation (%) |
|---|
| Age in years (Mean ± SD) | 56.35 ± 6.77 |
| Age groups | |
| < 50 years | 18 (45.0%) |
| 51 to 60 years | 11 (27.5%) |
| > 60 years | 11 (27.5%) |
| Females | 21 (52.5%) |
| Age (in years) at Onset of Diabetes (Mean ± SD) | 44.72 ± 7.13 |
| Family History of Diabetes | 30 (75.0%) |
| Existing Hypertension | 35 (87.5%) |
| Duration of Diabetes | 11.63 ± 5.76 |
| BMI (Kg/m2) | 26.96 ± 4.59 |
| Overweight | 13 (32.5%) |
| Obese | 13 (32.5%) |
Efficacy Assessments
Reduction in HbA1c, FBG and PPBG: Addition of basal insulin was associated with significant reduction in FBG and PPG as well as in HbA1c [Table/Fig-2]. There was slight increase in weight and associated increase in BMI, but the difference compared to baseline was non-significant. Systolic and diastolic BP decreases but reduction in diastolic BP was significant as compared to baseline [Table/Fig-2].
Changes in clinical characteristics at week 12 from baseline.
| Characteristics | Baseline | Week 12 | p-value |
|---|
| Weight (Kg) | 70.19 ± 14.94 | 71.25 ± 15.41 | NS |
| BMI (Kg/m2) | 26.96 + 4.59 | 27.24 + 4.78 | NS |
| Systolic BP (mmHg) | 132.43 ± 10.93 | 127.4 ± 10.31 | NS |
| Diastolic BP (mmHg) | 87.98 ± 7.69 | 81.83 ± 4.52* | <0.001 |
| FBG (mg/dl) | 318.55 ± 75.01 | 115.6 ± 11.09* | <0.001 |
| PPBG (mg/dl) | 378.90 ± 76.29 | 167.50 ± 19.46* | <0.001 |
| HbA1c (%) | 11.54 ± 1.41 | 7.70 ± 1.04* | <0.001 |
BMI: Body Mass Index, FBG: Fasting Blood Glucose, PPBG: Post-prandial Blood Glucose*p<0.05, significant; NS: Non-significant
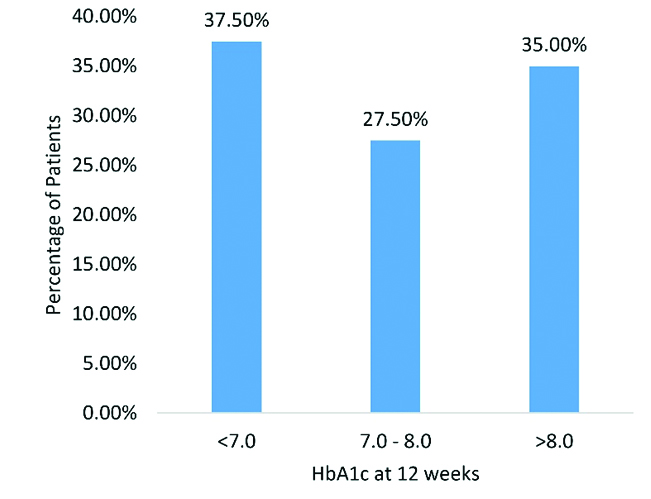
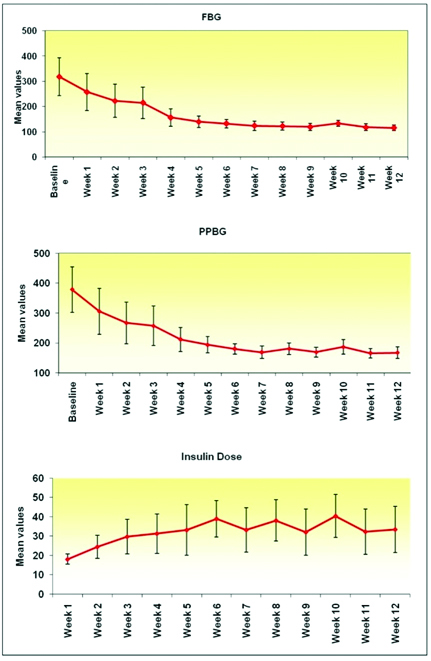
Goal HbA1c achievement: At week 12, 37.5% achieved goal HbA1c i.e., below 7.0%. Interestingly, 27.5% had HbA1c of 7% to 8% at week 12; whereas, rest were above 8% as depicted in [Table/Fig-3]. Weekly mean reduction in FBG and PPBG was in relation to insulin dose requirement as suggested in [Table/Fig-4]. Significant difference from baseline was found at week four for FBG and PPBG.
Achievement of goal HbA1c at week 12.
Weekly changes in FBG and PPBG and insulin doses.
Safety Assessments
There were 14 symptomatic and two nocturnal hypoglycaemia events during 12 week follow up. No cases of severe hypoglycaemia were found. No other treatment related adverse effects were reported.
Discussion
Basal insulin is among the second line options after metformin as recommended by the American Diabetes Association [6]. The problems of uncontrolled diabetes can be effectively managed with insulin treatment. Basal insulin provides safe and effective approach to intensive glycaemic management [11]. Insulin therapy is not restricted by age and is effective in young and elderly [12]. In our study, mean age of the patients was 56 years and over one-fourth were above 60 years. In a similar study from India by Singhai A et al., evaluating insulin glargine against premix insulin, mean age of the patients was 45 and 46 years in two groups respectively. Mean duration of diabetes in our study was 11.63 years [13]. Singhai A et al., reported mean diabetes duration of around 10 years [13]. In another study by Bretzel RG et al., mean duration of diabetes was eight and nine years in two study groups [8]. These data suggest that patients with T2DM taking OAAs eventually require insulin therapy after duration of 8 to 10 years, this point towards progressive loss of beta cell function. Nearly, two-third of the patients in our study were overweight and obese category. Previous studies also reported higher BMI which ranged in overweight and obese category [8,10,13]. Thus, the patients in our study had similar body compositions as compared to those which were studied previously.
A significant effect of insulin glargine started as basal insulin was evident on FBG and PPBG as well as on HbA1c. Significant reduction in glycaemic parameters suggests that the patients who fail on either one or two OAAs may derive benefits from once-a-day basal insulin. The dose of insulin was gradually increased over six weeks followed by some down-titration and up-titrations in remaining six weeks [Table/Fig-2] according to glycaemia improvements. Initiating basal insulin is recommended amongst 2nd line options after metformin by recent ADA guidelines [6]. Benefit of basal insulin may be more if started initially instead of adding other OAAs. In evaluating similar hypothesis, Aschner P et al., [14] observed significantly greater reduction in HbA1c at 24 weeks with insulin glargine as compared to sitagliptin when given to insulin naïve patients uncontrolled on metformin alone. Though hypoglycaemia and weight gain were more with insulin glargine, no significant difference was reported for severe symptomatic and severe nocturnal hypoglycaemic episodes. Further, basal insulin glargine treatment may be beneficial in obese patients. Obese diabetic with metabolic syndrome is the phenotype established in Indians which is associated with insulin resistance [15]. A combination of metformin with insulin glargine was found to be superior to combination of sulfonylurea and insulin glargine in patients of T2DM uncontrolled on metformin and thiazolidinedione (TZD) [16].Thus, effective and early glycaemic lowering with insulin glargine may result in long term benefits. This is especially helpful in Indian diabetics who have phenotype with higher degree of insulin resistance. This was further substantiated by our finding of 37.5% patients achieving glycaemic target of HbA1c below 7% and 27.5% of the patients were from 7% to 8%. Aschner P et al., also observed 68% patients achieving HbA1c below 7% at the end of 24-week treatment [14].
In addition to glycaemic lowering, mean reduction in both systolic and diastolic BP of magnitude of -5.0 mmHg and -6.1 mmHg respectively was seen. Insulin glargine is reported to have neutral effect on blood pressure [17]. Our observation of BP reduction could be due to improvements in glycaemia and thereby improving endothelial function. Basal insulin treatment when added to metformin and lifestyle therapy in obese, T2DM patients has been reported to improve endothelial function [18]. The ORIGIN (Outcome Reduction with an Initial Glargine Intervention) trial [19]. In a large patient population (n=12537) who had CV risk factors and impaired glycaemic status or T2DM, insulin glargine was not associated with lower rates of CV outcomes when compared with standard care delivered over six years. However, lower rate of new-onset diabetes was reported.
Increase in weight and thereby BMI is inevitable with insulin therapy. However, the weight increase was modest from baseline to week-12 and was not statistically significant. But this could be due to short-duration of our study. A weight gain to the magnitude of 1.2 to 1.4 kg at six months and up to 3.9 kg at one year has been reported with insulin glargine [17]. In the ORIGIN trial, median increment in weight was 1.6 kg with insulin glargine [19]. We recognize the need for long-term clinical study to assess effects of insulin glargine on weight in Indian subset of patients.
Safety analysis revealed only 14 cases of symptomatic hypoglycaemia and two cases of nocturnal hypoglycaemia none of them being severe episodes. Insulin glargine is reported to have lower rates of nocturnal hypoglycaemia as compared to the Neutral Protamine Hagedorn (NPH) insulin. Also, being a peakless insulin, there is lower risk of sever hypoglycaemia with insulin glargine [20].
Limitation
Our study adds more to the existing evidence on efficacy and safety of insulin glargine in Indian setting. We found significant glycaemic efficacy over 12 weeks with lower rates of hypoglycaemia and modest weight gain. Limitations include inadequate number of patients, short-duration, and being a single centre study. Also, we involved only patients who failed on one or two OAAs. A comparative evaluation on patients failed on one, two and three OAAs may provide better insight in to the efficacy and safety of insulin glargine. Also, we did not identify patient subgroups who can derive maximum benefits from this basal insulin. Complete assessments of changes in lipid parameters, renal function and endothelial function would provide more information on effects of insulin glargine in metabolic syndrome predominant phenotype of Indian diabetics. These limitations were due to cost constraints as this study was not funded and was conducted at a single centre.
Conclusion
Insulin glargine as a once a day basal insulin is effective and safe over short term in uncontrolled diabetics receiving two OAAs therapy. Modest weight gain and hypoglycaemia should not limit the use of insulin glargine. A reduction in BP in our study need further evaluation. A large, long term, randomized, controlled trial is needed to completely assess benefits and risks of insulin glargine in Indian setting.