Salivary Duct Carcinoma (SDC) is a distinctive and clinically aggressive adenocarcinoma of salivary origin. It arises from the ductal epithelium, predominantly occurring in the major salivary glands, especially the parotid gland. Here, we report a case of an extensive salivary gland pathology involving the right side of face, possibly arising from the parotid gland in a 25-year-old male patient. On routine histopathology, the tumour mass revealed a papillary pattern of neoplastic ductal epithelial cells showing comedo-like-central necrosis. Immunohistochemical staining showed tumour cells in the infiltrative component to be diffusely immunopositive for cytokeratin-7 and Her-2, confirming the diagnosis of SDC. This paper presents a case report on salivary duct carcinoma and highlights a review on histological variants of salivary duct carcinoma.
Case Report
A 25-year-old male patient reported to the Department of Oral Medicine with the chief complaint of a swelling on the right side of the face and neck. The patient had noticed the swelling seven months back in the right parotid region when it was small in size. Since then the swelling had progressively increased in size. There was no history of bleeding or pain associated with the swelling. Past medical history was not significant.
The patient was well built and had normal gait and posture. He was well oriented to surroundings and had normal vision and normal intelligence. His vital signs were within normal limits.
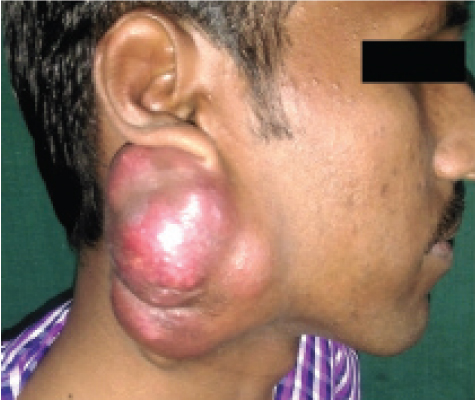
On extraoral examination an irregular multinodular swelling was noted on the right side of the face and neck [Table/Fig-1]. Antero-posteriorly, it measured 7 cm and superior-inferiorly 10 cm. The lobule of the right ear was raised. The skin over the swelling appeared shiny, stretched and erythematous. At the center, the nodules showed secondary changes and punctum formation. On palpation swelling was found to be non-tender, soft to firm in consistency, non-fluctuant, non-compressible and non-reducible. Temperature over the swelling was raised. The right submandibular lymph nodes were enlarged, firm and tender on palpation. Intraorally, no abnormality was detected related to the swelling.
Multinodular swelling on the right side of face with the right ear lobule raised.
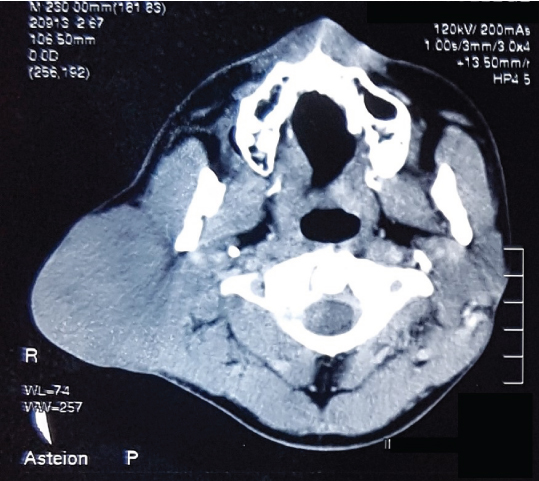
A CT scan was performed [Table/Fig-2] which confirmed the presence of a hyperdense lesion arising from the right parotid gland.
CT scan reveals a diffuse lobulated mass arising from the right parotid gland.
The tumour was clinically and radiologically staged as Stage III (T3N1M0) and a provisional diagnosis of malignant parotid gland tumour was given.
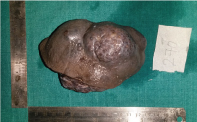
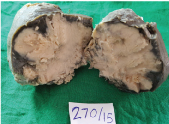
Surgical resection of the parotid tumour along with skin [Table/Fig-3] and lymph node dissection was performed. Grossly, the resected tumour was an irregular ovoid nodular mass of 11 x 8 cm. The cut surface showed a greyish white mass with small areas of necrosis as seen in the [Table/Fig-4].
Gross surgical resected specimen.
Gross image showing cut surface of specimen exhibiting encapsulated yellowish white mass.
Histopathological examination revealed multinodular growth pattern with islands of tumour cells showing central comedo-necrosis as seen in [Table/Fig-5]. Irregular papillary cystic arrangements with tumour cells that partially or completely filled cyst like spaces were also evident. The ductal epithelium showed fenestrations resembling Roman bridge architecture as shown in [Table/Fig-6]. The cells exhibited fine granular dense eosinophilic cytoplasm with pleomorphic nuclei. Squamous foci were also seen. The excised lymph nodes showed follicles replaced by tumour cells. Based on the above histopathological findings an impression of high grade salivary duct carcinoma was given.
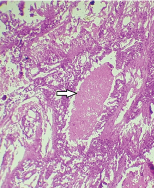
Photomicrograph showing necrosis seen intraductally resembling comedo-necrosis (arrow) (H&E, 10X).
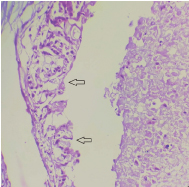
Photomicrograph showing ductal epithelium exhibiting fenestrations resembling Roman bridge architecture (arrow) (H&E stain, 40X).
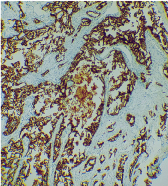
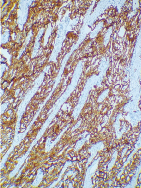
For the confirmation of the diagnosis, fresh sections of the tissue were further subjected to a panel of immunohistochemical markers. The tumour cells were diffusely immunopositive for cytokeratin 7 (CK7) [Table/Fig-7] and human epidermal growth factor (Her-2) [Table/Fig-8]. The tumour was immunonegative for CK14, CK20, p63 and GFAP. Intracytoplasmic mucin was absent. Finally, this lesion was diagnosed as SDC.
Photomicrograph showing tumour cells immunopositive for CK 7 (IHC 10X).
Photomicrograph showing tumour cells immunopositive for Her-2 (IHC 10X).
The management included wide surgical excision of the tumour followed by chemotherapy and radiotherapy. The superficial lobe and part of the deep lobe were removed and a small portion of parotid gland was left behind. All the branches of the facial nerve were preserved. The patient was on regular follow up after surgery and was undergoing chemotherapy and radiotherapy, but the patient eventually died after a period of 10 months.
Discussion
Salivary duct carcinoma is a rare, aggressive high grade salivary gland malignancy, accounting for about 0.2%-2% of all the salivary gland neoplasms [1]. Salivary duct carcinoma was identified as a distinct clinicopathologic entity by Kleinsasser and his colleagues in the year 1968 as a salivary gland adenocarcinoma [2]. It bears a marked histological resemblance to the invasive ductal carcinoma of the breast and prostate [3]. The term SDC was proposed based on its resemblance to ductal carcinoma of breast and was also sanctioned by WHO in the year 1991 [4].
The tumour has a predilection for male gender and the age of presentation ranges from 22 to 91 years, but most patients are older than 50 years [5]. The present case report is of a young patient aged 25 years.
Salivary duct carcinoma arises ab initio (from the beginning) or may develop in a pre-existing pleomorphic adenoma [3]. The tumour is said to be originated from the excretory and interlobular ducts of the major salivary glands [6]. Malignant transformation of ductal epithelial cells could be the reason for a salivary duct carcinoma arising in a pre-existing pleomorphic adenoma. In one case, reported in the literature, multifocal origin from major excretory ducts surrounding a pleomorphic adenoma was observed [7]. The present case reported here arose as ab initio tumour.
This tumour has a propensity for infiltrating into the surrounding tissues and frequently shows haematogenous and lymphatic metastasis [4]. Salivary duct carcinoma has an aggressive clinical course and a poor prognosis; most series have shown that more than 70% of patients die within three years of disease [3]. In our reported case, similar findings were observed and the patient died within one and a half years of its detection.
Salivary duct carcinoma is usually a high-grade malignancy, but Low Grade Salivary Duct Carcinoma (LG-SDC) is also reported in literature. The low-grade variant is usually a small lesion and grossly shows a cystic appearance. Histologically, it shows an intraductal proliferation resembling atypical breast ductal hyperplasia or in situ micropapillary ductal carcinoma [1]. The present case exhibited a high-grade malignancy.
Salivary duct carcinoma presents with a vast range of histologic variants. The main microscopic feature of this tumour is intraductal or circumscribed nests of dysplastic ductal cells that grow in solid, cribriform or papillary patterns [2,4,8,9]. The tumour cells exhibit central comedo-necrosis, which is a characteristic finding of salivary duct carcinoma. The intraductal component exhibits expanded salivary ducts without a definite lobular arrangement showing fenestrated Roman bridge appearance [4,8,9].
Histopathologically, salivary duct carcinoma exhibits three common variants; these are sarcomatoid, mucin rich and invasive micropapillary [5,3,9].
Sarcomatoid salivary duct carcinoma presents a dimorphic pattern showing presence of both carcinomatous and sarcomatoid components [5]. A transitional zone was distinctly recognized between the carcinomatous and mesenchymal components [10]. The carcinomatous and sarcomatoid areas may vary in proportion. Nagao T et al. reported eight cases of sarcomatoid salivary duct carcinoma, of which six cases showed predominating sarcomatoid areas [11].
Mucin rich variant demonstrates pools of mucin which surround the islands of tumour cells [5]. These floating tumour nests show conventional salivary duct carcinomatous elements with frequent comedo-necrosis. The mucin areas are in turn separated into compartments by fibrous connective tissue. The presence of small quantities of mucin have often been described in salivary duct carcinoma, but not large extracellular mucinous pools, which are prominent in mucin rich variant. According to the definition by Simpson RHW et al., when more than 40% of areas consist of mucinous components, mucin rich salivary duct carcinoma can be diagnosed [12]. Terasaki M et al., reported a case of mucin rich salivary duct carcinoma of submandibular gland which demonstrated 95% mucinous component [13].
Invasive micropapillary variant is characterized by foci where tumour cells form morula like papillary cell clusters which lack fibrovascular cores and are surrounded by clear spaces. Focal squamous differentiation adjacent to micropapillary component was reported in one case of invasive micropapillary salivary duct carcinoma. Invasive micropapillary variant has a more aggressive clinical course than conventional salivary duct carcinoma. It is associated with frequent lymphatic invasion and lymph node metastasis [5]. Nagao T et al., reported 14 cases of invasive micropapillary component in which all cases showed lymphatic metastasis and nine cases showed distant metastasis [14].
The case reported here exhibited a papillary growth pattern with focal squamous differentiation present adjacent to the micropapillary component suggestive of an invasive micropapillary variant of salivary duct carcinoma.
Immunohistochemical staining can be of greater value in confirming the diagnosis of salivary duct carcinoma. SDC is immunoreactive for low molecular weight cytokeratins, epithelial membrane antigen and androgen receptors [1,9]. The positive expression of CK7 in the tumour cells is supported by most studies on SDCs. About 90% of reported cases of SDC have shown immunopositive staining for HER-2/neu. Over expression of this protein aids in predicting the risk for local invasion, recurrence, distant metastasis and thereby poor prognosis [15].
The primary tumours of breast and prostate may metastasize to the salivary glands and pose difficulty in diagnosis [6]. Most of the salivary gland malignancies show positive immunostaining for CK7. Immunoexpression of CK7 may provide valuable information in the differential diagnosis of primary salivary gland carcinomas and metastatic tumours [9]. Immunohistochemical expression of oestrogen and progesterone receptor confirms the presence of metastatic breast carcinoma [14]. Metastatic prostrate carcinoma will show positive expression for Prostrate Specific Antigen (PSA) and prostrate acid phosphatase [6,14]. Positivity of these markers is rare in primary salivary duct carcinoma. SDC are usually negative for expression of S-100, p63 and smooth muscle myosin [1].
In the present case, the immunohistochemistry showed positive for CK7 and Her-2. CK7 positivity confirmed the glandular origin of the tumour. Previous reported cases and review of literature suggests that expression of Her-2 has been associated with unfavorable clinical outcome [15]. This finding was consistent in this case, as the present patient died 10 months after initial surgery followed by chemotherapy and radiotherapy. The above factors seen in the present case indicates the aggressiveness and poor prognosis of salivary duct carcinoma.
Conclusion
A case of a high grade SDC in a 25-year-old male patient is being reported here with highlighting the review of literature along with special emphasis on histopathology and immunohistochemistry. Even after the early detection, diagnosis and treatment, the patient died after 10 months post-surgery. This sheds light on the aggressiveness and poor prognosis of this tumour.
[1]. Xie S, Yang H, Bredell M, Shen S, Huijun Y, Jin L, Salivary duct carcinoma of the parotid gland: A case report and review of the literatureOncology letters 2015 9:371-74. [Google Scholar]
[2]. Afzelius L, Cameron WR, Svensson C, Salivary duct carcinoma clinicopathologlc study of 112 casesHead & Neck Surgery 1987 9:151-56. [Google Scholar]
[3]. Slootweg PJ, Cardesa A, Pathology of head and neck 2006 Springer-Verlag:154-156. [Google Scholar]
[4]. Gondak RO, Mariano FV, Alves FA, Almeida OP, Lopes MA, Salivary duct carcinoma in minor salivary glands: report of two cases with different clinical behaviorJ Clin Exp Pathol 2014 4(2):1-6. [Google Scholar]
[5]. Thompson LDR, Head and neck pathology 2013 2nd edChinaElseiver:304-310. [Google Scholar]
[6]. Simpson RHW, Clarke TJ, Sarsfield PTL, Babajews AV, Salivary duct adenocarcinornaHistopathology 1991 18:229-35. [Google Scholar]
[7]. Delgado R, Vuitch F, Saavedra JA, Salivary duct carcinomaCancer 1993 72(5):1503-12. [Google Scholar]
[8]. Acharya S, Padmini S, Koneru A, Krishnapillai R, Intraoral salivary duct carcinoma: A case report and a brief reviewJ Oral Maxillofac Pathol 2014 18(1):121-27. [Google Scholar]
[9]. Chandrasekar C, Salati N, Rao L, Radhakrishnan R, Salivary duct carcinoma in the mandibular anterior region: The role of immunohistochemical markers in its definitive diagnosisJ Oral Maxillofac Pathol 2016 20:505-09. [Google Scholar]
[10]. Ide F, Mishima K, Saito I, Sarcomatoid salivary duct carcinoma of the oral cavityVirchows Arch 2003 443:686-89. [Google Scholar]
[11]. Nagao T, Gaffey TA, Serizawa H, Iwaya K, Watanabe A, Yoshida T, Sarcomatoid variant of salivary duct carcinomaAm J Clin Pathol 2004 122:222-31. [Google Scholar]
[12]. Simpson RHW, Prasad AR, Lewis JE, Skalova A, David L, Mucin- rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four casesAm J Surg Pathol 2003 27:1070-79. [Google Scholar]
[13]. Terasaki M, Terasaki Y, Wakamatsu K, Takahashi M, Kunugi S, Urushiyama H, A mucin-rich variant of salivary duct carcinoma with a prominent mucinous component, a tumour that mimics mucinous adenocarcinomaOral Surg Oral Med Oral Pathol Oral Radiol 2013 116:210-14. [Google Scholar]
[14]. Myers EN, Ferris RL, Salivary gland disorders 2007 GermanySpringer-Verlag Berlin Heidelberg:76-78. [Google Scholar]
[15]. Skalova A, Starek I, Kucerova V, Szepe P, Plank L, Salivary duct carcinoma – a highly Aggressive salivary gland tumour with her-2/neu Oncoprotein overexpressionPathol Res Pract 2001 197:621-26. [Google Scholar]