Introduction
Oral cancer is a major global threat to public health. It is one of the most common causes of mortality and morbidity in the modern era. Oral Squamous Cell Carcinoma (OSCC) accounts for over 90% of the malignancies involving the oral cavity. The enzyme- Butyryl Cholinesterase (BChE) is proposed to have a role in cell proliferation, cell adhesion, cell differentiation, apoptosis and tumorigenesis. Few studies have been conducted for understanding the significance of serum BChE as a biomarker in oral cancer patients; however literature available is insufficient to arrive at a conclusion. There is a need of a simple, rapid, convenient, inexpensive and reliable biomarker of oral cancer. So, the present study is an attempt to estimate the level of BChE in oral cancer, prior to definitive therapy.
Aim
To estimate and compare the serum BChE levels in patients with OSCC with age and gender matched healthy controls.
Materials and Methods
The study comprised of 80 subjects, of which 40 biopsy proven OSCC patients of either sex were selected as cases and 40 healthy, age and gender matched subjects as controls. Estimation of serum BChE levels was done by colorimetric method using RANDOX RX Imola Auto-Analyzer. The statistical analysis between the OSCC group and the control group were done using unpaired t-test. Comparison between serum BChE levels and TNM stages of OSCC were done using Kruskal-Wallis Test. Comparison between serum BChE levels and histopathological grades of OSCC were done using Mann-Whitney U Test.
Results
There was statistically highly significant decrease in the mean serum BChE levels in the OSCC group compared to the control group (p<0.001). It was revealed that the serum BChE levels were further decreased in moderately differentiated squamous cell carcinoma than well differentiated squamous cell carcinoma and the difference was statistically significant (p <0.05).
Conclusion
The decrease in the serum BChE level demonstrates that it as a simple, rapid, convenient, inexpensive and reliable biomarker for oral cancer. Our findings support the concept of role of BChE in apoptosis, cell proliferation, differentiation and its related link in the pathophysiology of oral cancer.
Introduction
Oral cancer development is a multistep and multifocal process which involves field cancerization and carcinogenesis [1]. Biochemical changes in tissues provide a better understanding of the chemical processes responsible for malignancy and also aid in early cancer detection. These biochemical changes can be studied by advanced molecular biology and enzymology [2]. The role of Acetylcholinesterase (AChE) in terminating acetylcholine-mediated neurotransmission made it the focus of intense research for much of the past century. But the complexity of AChE gene regulation and recent evidence for some of the suspected non-classical actions of AChE enzyme has more recently led to renewed interest in AChE research [3]. Both AChE and BChE have been proposed to have a role in lipoprotein metabolism, myelin maintenance, scavenging of toxic molecules, in the processing of the amyloid precursor protein, neuritogenesis, synaptogenesis, hematopoiesis, thrombopoiesis, cell growth, cell adhesion, cell differentiation, apoptosis and tumorigenesis [4-6]. Overexpression of cholinesterase was shown to inhibit cell proliferation and promote apoptosis in the cells [7]. The aim of this study was to estimate the serum BChE in OSCC patients prior to definitive therapy.
Materials and Methods
A cross-sectional study was conducted in the Department of Oral Medicine and Radiology, Bapuji Dental College and Hospital, Davangere, Karnataka, India, after obtaining approval from the Institutional Ethical Committee. The time period of the study was one year. Based on the inclusion and exclusion criteria, a total of 80 subjects were included for the study.
Sample size (n) was determined using the following formula:
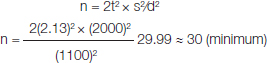
[t2=theoretical value of distribution, s= pooled standard deviation, d= mean expected difference (i.e. mean expected difference in BChE levels between in subjects and controls]
A total of 40 clinical and biopsy proven OSCC patients of either sex were selected as study group and 40 healthy age and gender matched subjects were selected as control group. TNM classification was used to stage OSCC clinically and Broder’s (1920) grading system was used to stage OSCC histopathologically [8,9]. Only those patients who gave a signed informed consent form voluntarily were allowed to participate in the present study.
Patients suffering from any systemic diseases, history of chemotherapy and radiotherapy, patients with any other malignancy other than OSCC, recurrent cases of OSCC, and patients under medication with depolarising muscle relaxants, anti-cholinesterases, and oral contraceptives were excluded from the study. For the control group, in addition to the above criteria, subjects with any habit of tobacco and alcohol were excluded.
Under aseptic conditions 2 ml venous blood was withdrawn from each individual using a sterile disposable syringe. The blood drawn was transferred to a vacutainer without anticoagulant. The sample was allowed to clot for 30 minutes. After centrifugation at 3000 rpm for five minutes, clear supernatant serum was obtained. The quantitative in-vitro determination of BchE in serum was done using colorimetric method. The clear supernatant serum obtained after centrifugation was used for the determination of BChE levels by spectrophotometric method [10] using a Randox RX Imola Autoanalyzer.
Statistical Analysis
The obtained data was analyzed by using the software – statistical package for social science version-18.0 (SPSS-18). Statistical analysis was done using unpaired t test, Kruskal Wallis and Mann- Whitney U test.
Results
The study consisted of a total of 80 subjects which comprised of 40 biopsy proven OSCC patients and 40 controls. Both the groups comprised of 26 (65%) males and 14 (35%) females. The age of the subjects ranged from 32- 73 years with a mean age of 54.6 years (standard deviation of 12.4). Based on clinical examination, TNM classification was used to stage OSCC. It was observed that 6 (15%) patients were in Stage I, 15 (37.5%) patients in Stage II, 14 (35%) patients in Stage III and 5 (12.5%) patients were in Stage IVA of TNM staging. Broder’s (1920) grading system was used to stage the squamous cell carcinoma histopathologically. It was observed that out of 40 cases, 15 (37.5%) patients had well differentiated squamous cell carcinoma and 25 (62.5%) patients had moderately differentiated squamous cell carcinoma at the time of presentation. None of the OSCC patients had poorly differentiated or anaplastic grades histopathologically.
The mean value of serum BChE was 6708.1533 U/L for OSCC cases whereas, in controls the mean value of serum BChE was 8582.8108 U/L. Unpaired t test was used to check the difference in mean between the two groups. There was a statistically significant difference (p<0.001) between the mean serum BChE levels of the two groups [Table/Fig-1].
Comparison of serum BChE levels between study and control group.
| Study Groups | Number of subjects | Mean serum BChE Level in (U/L) | Standard Deviation | Standard Error Mean | Significancep-value |
|---|
| Cases | 40 | 6708.15 | 1040.77 | 164.56 | <0.001Highly significant |
| Controls | 40 | 8582.81 | 1002.31 | 158.47 |
Unpaired t-test.
It was observed that mean serum BChE level in well differentiated squamous cell carcinomas was 7099.0480 U/L whereas, in moderately differentiated squamous cell carcinoma, it was 6473.6164 U/L. Mann Whitney U test was used to check the difference in mean between the two groups. There was statistically significant difference between the two groups (p<0.05) [Table/Fig-2].
Comparison of mean serum BChE levels in different histopathologic stages.
| Histopathology | Number of cases | Mean serum BChE Level in U/L | Standard deviation | Standard error | Significance p-value |
|---|
| Well Differentiated SCC | 15 | 7099.05 | 1184.41 | 305.82 | < 0.05Significant |
| Moderately Differentiated SCC | 25 | 6473.62 | 888.32 | 177.66 |
Mann-Whitney U Test.
It was also observed that the mean serum BChE level for TNM stage I was 6726.3667 U/L, TNM stage II was 6608.3433 U/L, TNM stage III was 7025.0100 U/L, and TNM stage IVA was 6098.5280 U/L. Kruskal Wallis test was used to check the difference in mean between the four groups. The mean serum BChE decreased from Stage I to Stage IV a (except for stage III) but the difference was statistically not significant (p>0.05) [Table/Fig-3].
Comparison of mean serum BChE levels in different TNM stages of OSCC.
| Staging | Mean serumBChELevel in U/L | Number ofcases | StandardDeviation | Significancep-value |
|---|
| Stage I | 6726.37 | 6 | 1417.38 | >0.05(Not significant) |
| Stage II | 6608.34 | 15 | 670.38 |
| Stage III | 7025.01 | 14 | 1250.44 |
| Stage IV A | 6098.53 | 5 | 735.99 |
| Total | 6708.1533 | 40 | 1040.77472 |
Kruskal–Wallis test.
Discussion
The burden of cancer is growing globally and is one of the top leading causes of death. Early diagnosis of oral OSCC is still challenging for clinicians and patients. Biochemical markers as an adjunct for diagnosis and for predicting prognosis of disease is an area of developing research. Each cell type has a unique molecular imprint, known as biomarkers. With the advances in molecular biology and clinical enzymology, changes in the malignant cell can be explained on the basis of biochemical changes in the tissue. These biomarkers can be of great benefit in the management of oral cancer [11,12].
Two types of cholinesterase have been recognized in the mammalian system, the true cholinesterase or Acetylcholinesterase (AChE) and Pseudocholinesterase (PChE). The enzyme, PChE is also known as serum cholinesterase or BChE [4]. Though, these two enzymes are products of different genes on human chromosomes 7 (specifically 7q22) and 3 (specifically 3q26), respectively, they share 65% similar amino acid sequence and molecular forms [13,14]. The AChE is responsible for hydrolysis of acetylcholine at neuromuscular junction efficiently whereas BChE has comparatively less affinity for acetylcholine. BChE has preference for butyrylcholine rather than other choline and esters [15].
Though, the literature available regarding the role of BChE enzyme in oral cancer is limited, there is increasing evidence supporting the involvement of cholinesterase in tumorigenesis. Studies conducted in various human cancers have shown an alteration in BChE activity. Most of these studies have demonstrated a decrease in the levels of this enzyme in blood, [7,16-23] whereas contradicting this; few studies have reported an increase in the enzyme level in cancer [2,10,24-27] [Table/Fig-4,5]. The studies which favoured the increase in cholinesterase activity in cancer could not give a satisfactory explanation for the elevation in the enzyme activity.
Studies demonstrating a decrease in serum BChE levels in cancer.
| Study | Site of cancer |
|---|
| Montenegro MF et al., 2006 [7] | Colorectal |
| Chougule A et al., 2008 [16] | Head and neck |
| Bradamante V et al., 2000 [17] | Uterine cervix |
| Ruiz-Espejo F et al., 2002 [18] | Breast |
| Ghooi AM et al., 1980 [19] | Multiple sites |
| Sen R et al., 1987 [20] | Oral cavity |
| Barbosa M et al., 2001 [21] | Brain |
| Battisti V et al., 2012 [22] | Prostrate |
| Lapidot-Lifson Y et al., 1989 [23] | Blood |
Studies demonstrating an increase in serum BChE levels in Cancer.
| Study | Site of cancer |
|---|
| Prabhu K et al., 2011 [2] | Oral cavity |
| Chianeh YR et al., 2014 [10] | Oral cavity |
| Muñoz-Delgado E et al., 2008 [24] | Kidney |
| Nieto-Cerón S et al., 2010 [25] | Prostrate |
| Zanini D et al., 2013 [26] | Lung |
| Mikecin L et al., 2013 [27] | CNS |
The drop in cholinesterase activity is said to have a vital pathophysiological role in tumorigenesis. The non-classical roles of cholinesterase have been implicated in modulating cancer growth. The exact role of cholinesterase in oncogenesis or tumor progression is still unclear. But it has been suggested that cholinesterase genes (AChE and BChE) have shown structural alteration and their protein products have exhibited aberrant expression in a variety of tumor types [28]. Interestingly a number of studies have shown a genetic alterations in the long arm of chromosome 7 (7q22), the genetic locus of the AChE gene, in several human cancers [29-35].
Cell proliferation, differentiation and death are important biological processes in multicellular organisms, and evidence suggests a link between apoptosis and proliferation. Over expression of cholinesterase was shown to inhibit cell proliferation and promote apoptosis in cells [36]. At the same time, the down regulation of AChE expression with inhibited apoptosis suggest that AChE is potentially a marker and a regulator of apoptosis. The defective cholinergic signalling may alter the activities of cdc 2- like kinases with the potential to accelerate the rate of cell division and support tumoral growth [37]. These data raise the intriguing possibility that the pro apoptotic role of AChE could play a role in tumor suppression [38].
Comparison between histopathological groups has demonstrated a statistically significant (p<0.05) decrease in serum BChE levels in moderately differentiated OSCC compared to well differentiated OSCC. This probably indicates drop of enzyme with progression of the disease.
There was a gradual decrease in the mean serum BChE level from stage I to stage IVA except for stage III where the level was spiked above the stage I. But it did not demonstrate any statistically significant (p>0.05) difference between mean levels of BChE in different TNM stages. Similiarly, Chougule A et al., (2008) and Sen R et al., in oral cancer (1987) in head and neck cancers (except for stage II) found diminishing activity of BChE activity with advancement in the clinical staging [16,20].
The decrease in the BChE level not only demonstrates it as a possible biomarker of oral cancer, but further supports the concept of role of BChE in apoptosis, cell proliferation, differentiation and its related link in pathophysiology of cancer. Further in- depth research needs to be undertaken regarding the reliability of BChE being considered as a dependable prognostic biomarker of oral cancer.
Limitation
There were a few limitations to our study such as a small sample size, unequal male: female ratio, and there was no distribution of the subjects by the age.
Conclusion
Our study demonstrated a highly significant decrease in the serum levels BChE in OSCC patients compared to controls. We conclude that the decrease of serum BChE enzyme levels can be used a reliable biomarker for oral cancer, which certainly indicates the progression of disease. All these data support the hypothesis that cholinesterase genes could function as a tumor suppressor gene and BChE could be a reliable marker of carcinogenesis. In addition, there is an established clinical importance of decrease in the serum levels of BChE or PChE in anaesthesia. The decrease of this particular enzyme levels in oral cancer patients is again a concern as they are most often candidates of surgical interventions.
Unpaired t-test.
Mann-Whitney U Test.
Kruskal–Wallis test.
[1]. Tanaka T, Ishigamori R, Understanding carcinogenesis for fighting oral cancerJ Oncol 2011 2011:603740 [Google Scholar]
[2]. Prabhu K, Naik D, Ray S, Vadiraj Rao A, Kamath A, Significance of serum butyrylcholinesterase levels in oral cancerAMJ 2011 4(7):374-78. [Google Scholar]
[3]. Soreq H, Seidman S, Acetylcholinesterase—new roles for an old actorNat Rev Neurosci 2001 2(4):294-302. [Google Scholar]
[4]. Patocka J, Kuca K, Jun D, Acetylcholinesterase and butyrylcholinesterase—important enzymes of human bodyActa Medica (Hradec Kralove) 2004 47(4):215-28. [Google Scholar]
[5]. Wessler I, Kirkpatrick CJ, Racké K, Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humansPharmacol Ther 1998 77(1):59-79. [Google Scholar]
[6]. Zhang XJ, Yang L, Zhao Q, Caen JP, He HY, Jin QH, Induction of acetylcholinesterase expression during apoptosis in various cell typesCell Death Differ 2002 9(8):790-800. [Google Scholar]
[7]. Montenegro MF, Ruiz-Espejo F, Campoy FJ, Muñoz-Delgado E, de la Cadena MP, Rodríguez-Berrocal FJ, Cholinesterases are down-expressed in human colorectal carcinomaCell Mol Life Sci 2006 63(18):2175-82. [Google Scholar]
[8]. Broders AC, Squamous-cell epithelioma of the lip. A study of five hundred and thirty-seven casesJAMA 1920 74(10):656-64. [Google Scholar]
[9]. Epstein JB, Oral cancer. In: Greenberg MS, Glick M and Ship JABurket’s Oral Medicine, Diagnosis and Treatment 2008 11th edCanadaBC Decker Inc:153-191. [Google Scholar]
[10]. Chianeh YR, Manjunath R, Prabhu K, Fernandes D, Vidyasagar M, Kamath A, Protein thiols and butryrylcholinestrase in saliva of oral cancer patientsIndian J Clin Biochem 2014 29(2):238-41. [Google Scholar]
[11]. Ludwig JA, Weinstein JN, Biomarkers in cancer staging, prognosis and treatment selectionNat Rev Cancer 2005 5(11):845-56. [Google Scholar]
[12]. Nagler RM, Saliva as a tool for oral cancer diagnosis and prognosisOral Oncol 2009 45:1006-10. [Google Scholar]
[13]. Allderdice PW, Gardner HA, Galutira D, Lockridge O, LaDu BN, McAlpine PJ, The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26Genomics 1991 11(2):452-54. [Google Scholar]
[14]. Cokuğraş AN, Butyrylcholinesterase: structure and physiological importanceTurk J Biochem 2003 28:54-61. [Google Scholar]
[15]. Silman I, Sussman JL, Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacologyCurr Opin Pharmacol 2005 5(3):293-302. [Google Scholar]
[16]. Chougule A, Hussain S, Agarwal DP, Prognostic and diagnostic value of serum pseudocholinesterase, serum aspartate transaminase, and serum alanine transaminase in malignancies treated by radiotherapyJ Cancer Res Ther 2008 4(1):21-25. [Google Scholar]
[17]. Bradamante V, Smigovec E, Bukovic D, Geber J, Matanic D, Plasma cholinesterase activity in patients with uterine cervical cancer during radiotherapyColl. Antropol 2000 24(2):373-80. [Google Scholar]
[18]. Ruiz-Espejo F, Cabezas-Herrera J, Illana J, Campoy FJ, Vidal CJ, Cholinesterase Activity and Acetylcholinesterase Glycosylation are altered in Human Breast CancerBreast Cancer Res Treat 2002 72(1):11-22. [Google Scholar]
[19]. Ghooi AM, Malaviya GN, Kashyap A, A comparative study of LDH & PchE in sera of cancer patients; a preliminary reportIndian J Cancer 1980 17(1):31-33. [Google Scholar]
[20]. Sen R, Sur R, Dasgupta R, Mazumder GC, Serum pseudocholinesterase activity & protein bound fucose level in oral malignancyIndian J Cancer 1987 24(4):242-50. [Google Scholar]
[21]. Barbosa M, Rios O, Velásquez M, Villalobos J, Ehrmanns J, Acetylcholinesterase and butyrylcholinesterase histochemical activities and tumor cell growth in several brain tumorsSurg Neurol 2001 55(2):106-12. [Google Scholar]
[22]. Battisti V, Bagatini MD, Maders LD, Chiesa J, Santos KF, Gonçalves JF, Cholinesterase activities and biochemical determinations in patients with prostate cancer: influence of Gleason score, treatment and bone metastasisBiomed Pharmacother 2012 66(4):249-55. [Google Scholar]
[23]. Lapidot-Lifson Y, Prody CA, Ginzberg D, Meytes D, Zakut H, Soreq H, Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesisProc Natl Acad Sci U S A 1989 86(12):4715-19. [Google Scholar]
[24]. Muñoz-Delgado E, Montenegro MF, Morote-García JC, Campoy FJ, Cabezas-Herrera J, Kovacs G, The expression of cholinesterases in human renal tumors varies according to their histological typesChem Biol Interact 2008 175(1-3):340-42. [Google Scholar]
[25]. Nieto-Cerón S, Vargas-López H, Pérez-Albacete M, Tovar-Zapata I, Martínez-Hernández P, Rodríguez-López JN, Analysis of cholinesterases in human prostate and sperm: implications in cancer and fertilityChem Biol Interact 2010 187(1-3):432-35. [Google Scholar]
[26]. Zanini D, Schmatz R, Pelinson LP, Pimentel VC, da Costa P, Cardoso AM, Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancerMol Cell Biochem 2013 374(1-2):137-48. [Google Scholar]
[27]. Mikecin L, Krizmaric M, Stepan Giljevic J, Gjurasin M, Kern J, Lenicek Krleza J, Pseudocholinesterase activity in cerebrospinal fluid as a biomarker of solid central nervous system tumors in childrenCroat Med J 2013 54(5):429-35. [Google Scholar]
[28]. Dich J, Zahm SH, Hanberg A, Adami HO, Pesticides and cancerCancer Causes Control 1997 8(3):420-43. [Google Scholar]
[29]. Neville PJ, Thomas N, Campbell IG, Loss of heterozygosity at 7q22 and mutation analysis of the CDP gene in human epithelial ovarian tumorsInt J Cancer 2001 91(3):345-49. [Google Scholar]
[30]. Zeng WR, Watson P, Lin J, Jothy S, Lidereau R, Park M, Refined mapping of the region of loss of heterozygosity on the long arm of chromosome 7 in human breast cancer defines the location of a second tumor suppressor gene at 7q22 in the region of the CUTL1 geneOncogene 1999 18(11):2015-21. [Google Scholar]
[31]. Lundgren R, Mandahl N, Heim S, Limon J, Henrikson H, Mitelman F, Cytogenetic analysis of 57 primary prostatic adenocarcinomasGenes Chromosomes Cancer 1992 4(1):16-24. [Google Scholar]
[32]. Takahashi S, Shan AL, Ritland SR, Delacey KA, Bostwick DG, Lieber MM, Frequent loss of heterozygosity at 7q31.1 in primary prostate cancer is associated with tumor aggressiveness and progressionCancer Res 1995 55(18):4114-19. [Google Scholar]
[33]. Fischer K, Brown J, Scherer SW, Schramm P, Stewart J, Fugazza G, Delineation of genomic regions in chromosome band 7q22 commonly deleted in myeloid leukemiasRecent Results Cancer Res 1998 144:46-52. [Google Scholar]
[34]. Johansson B, Mertens F, Mitelman F, Cytogenetic deletion maps of hematologic neoplasms: circumstantial evidence for tumor suppressor lociGenes Chromosomes Cancer 1993 8(4):205-18. [Google Scholar]
[35]. Ozisik YY, Meloni AM, Surti U, Sandberg AA, Deletion 7q22 in uterine leiomyoma. A cytogenetic reviewCancer Genet Cytogenet 1993 71(1):01-06. [Google Scholar]
[36]. La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E, Epidemiology and prevention of oral cancerOral Oncol 1997 33(5):302-12. [Google Scholar]
[37]. Soreq H, Lapidot-Lifson Y, Zakut H, A role for cholinesterases in tumorigenesis?Cancer Cells 1991 3(12):511-16. [Google Scholar]
[38]. Stephenson J, Czepulkowski B, Hirst W, Mufti GJ, Deletion of the acetylcholinesterase locus at 7q22 associated with myelodysplastic syndromes (MDS) and acute myeloid leukaemia (AML)Leuk Res 1996 20(3):235-41. [Google Scholar]