Immuno-Histochemical and Quantitative Study of Melanocytes and Melanin Granules in Oral Epithelial Dysplasia
Swapna Honwad1, SV Ravi2, Mandana Donoghue3, Manjiri Joshi4
1 Senior Lecturer, Department of Oral Patholology, KMCT Dental College, Mukkam, Kerala, India.
2 Reader, Department of Conservative Dentistry and Endodontics, KMCT Dental College, Mukkam, Kerala, India.
3 Oral Pathologist, Department of Oral Pathology and Microbiology, D. Center, Belgaum, Karnataka, India.
4 Oral Pathologist, Department of Oral Pathology, College of Dental Sciences, Davangere, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Swapna Honwad, Senior Lecturer, Department of Oral Patholology, KMCT Dental College, Mukkam-673602, Kerala, India.
E-mail: dr.swapnahonwad@gmail.com
Introduction
Oral Epithelial dysplasia (OED) is a potentially malignant disorder that is characterized by the presence of architectural and cytological changes. One of the prime factors responsible for the development of these lesions is the usage of tobacco. A variety of factors provide protective mechanism in order to prevent the effects of chemotoxic agents including tobacco products of which, melanin pigmentation is one of the vital elements.
Aim
Role of melanocytes in progression of OED has remained unclear, so the present study was done to evaluate density of melanocyte and melanin granules in different grades of epithelial dysplasia and to correlate both findings with different grades of epithelial dysplasia.
Materials and Methods
The study included 60 OED cases, of which three histopathogical sections were prepared from each block. The sections were stained with Hematoxylin and Eosin, Masson Fontana and Human Melanoma Black (HMB-45), an immunohistochemical stain. Quantification of melanin granules was evaluated under 40X magnification using arbitrary scale with micrometer square as, 0= Absence of melanin granules, 1= Rare and scattered melanin granules, 2= Dense but not aggregated melanin granules, 3= Dense and aggregated melanin granules. Density of melanocytes was evaluated under 10X magnification. Five consecutive fields were evaluated for melanocytes and melanin granules starting from the field of highest density.
Results
There was an insignificant increase in number of melanocytes and melanin granules in mild and moderate dysplasia compared to normal but significant reduction was observed in severe dysplasia.
Conclusion
The decrease in number of melanocytes and melanin granules was proportional to severity of epithelial dysplasia. This could be due to chronic irritation by chemical products leading to death of melanocytes.
Carcinoma in situ, Masson fontana, Smoking, Tobacco, Silver nitrate
Introduction
Dysplasia is a Greek term used in pathology refers to abnormal (dys) growth (plasia). It is characterised by the presence of architectural and cytological changes and considered as gold standard for assessment of potentially malignant disorders [1,2]. One of the principal factors responsible for the development of these lesions is the usage of tobacco in its various forms like smoking cigarette, bidi and snuff chewing [3,4].
Tobacco releases benzopyridine epoxides when it is burnt, which initiates pathogenesis of oral squamous cell carcinoma. These chemicals stimulate oral mucosal melanocytes, which are non-keratinocytes situated in basal epithelial layer. These melanocytes synthesize melanin pigment which binds to epoxides to prevent further deleterious effects on cells [5]. Hence, the variation in melanin pigmentation and melanocyte density is one of the defensive components against the chemotoxic products of tobacco.
The pigment melanin produced by specialized cells melanocytes, is most commonly responsible for colour of oral mucosa. Melanin is synthesized within melanocytes as small structures called melanosomes, which are inoculated in to cytoplasm of melanocytes and transferred to adjacent keratinocytes [6]. Melanocytes have a low proliferation potential and resist apoptosis as they are endowed with antiapoptotic mechanisms. And these cells have a very long life span, and survive for decades [6,7].
Melanin pigment producing cells are altered in various physiologic and pathologic conditions. Their role in health and disease has remained unclear which attracted further research. There is an absence of evidence related to melanocytes and their possible role in progression of OED. So, the present study was aimed at assessing the changes in density of melanocytes and melanin granules in different grades of epithelial dysplasia which can bring new auxiliary data about role of melanocytes and melanin in different grades of OED.
Materials and Methods
This retrospective study was carried out on 60 pregraded cases of OED of which 20 cases of mild, moderate and severe dysplasia were taken from the archives of Department of Oral Pathology and Microbiology, College of Dental Sciences, Davangere, Karnataka, India. The study was lacking details of age, gender and tobacco habits of each case since they were not properly available in archives. Normal oral mucosa were used as control. The proven cases of malignancies were excluded.
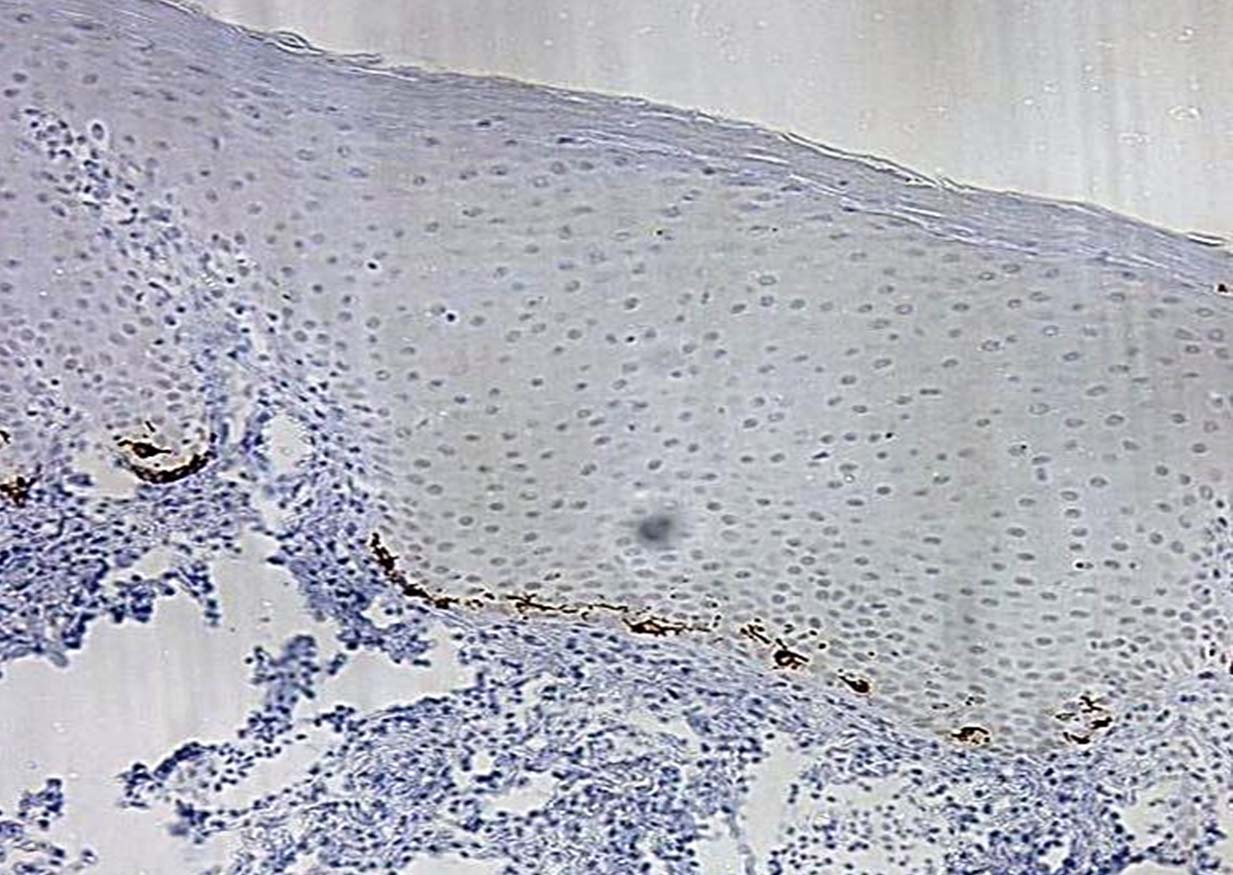
Two paraffin embedded tissue sections of 5 μm -6 μm and one section of 4 μm were prepared from each block using semiautomatic soft tissue microtome (Leica, Germany). Two sections were mounted on glass slide and stained with Harris Haematoxylin and Eosin (H&E) stain for regrading epithelial dysplasia [Table/Fig-1] and Masson Fontana’s special stain for melanin granules [Table/Fig-2]. Third section of 4 μm thickness was mounted on adhesive coated slides and stained with HMB-45 immunohistochemical staining kit (Bio genex private Ltd, USA), which detects particularly activated melanocytes [Table/Fig-3,4].
Haematoxylin and Eosin stained slide (10X) for grading epithelial dysplasia.
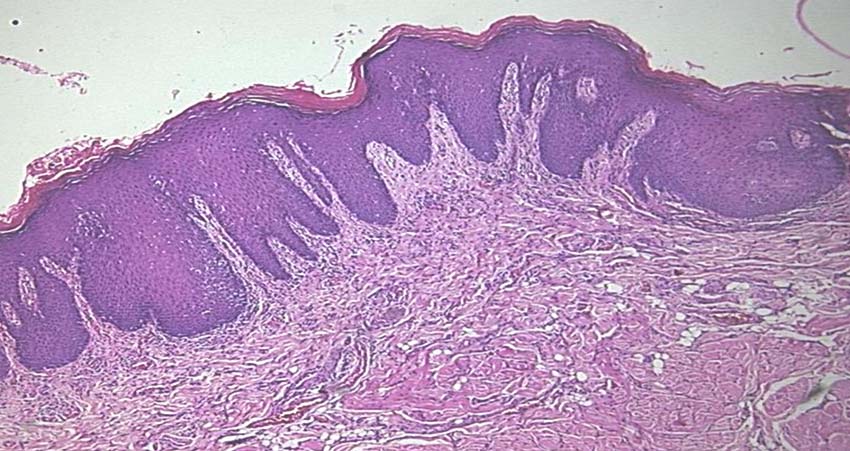
Melanin granules in epithelium stained with Masson Fontana (40 X).
HMB-45 immunohistochemical kit (Bio genex).

HMB-45 immunohistochemical stained slide (10X).
The H&E stained slides were graded as mild, moderate and severe epithelial dysplasia according to WHO 2005 criteria [8].
Presence and distribution of melanin granules was evaluated microscopically using micrometer square. (Grid)
The quantification of melanin granules was determined by measuring density of melanin granules in each micro squares on the basis of following arbitrary scale:
0= Absence of melanin granules.
1= Rare and scattered melanin granules.
2= Dense but not aggregated melanin granules.
3= Dense and aggregated melanin granules [9].
Five consecutive fields starting from highest density were evaluated under 40X magnification.
Immunohistochemically stained study slides were evaluated to count the melanocytes in five consecutive fields starting from field of highest density under 10X magnification.
Statistical Analysis
The data obtained from all stained slides were tabulated and statistically analysed. A p-value of 0.05 or less was considered statistically significant. Statistical methods used were, median, Kruskall Wallis and Mann Whitney U test. Since the obtained data highly skewed, median was preferred.
Results
The pregraded test slides were graded again to minimise subjective error, so there was disparity in sample size. After grading, 19 mild, 21 moderate and 20 severe dysplasia cases were established.
The obtained data showed mild increase in number of melanocytes among mild and moderate epithelial dysplasia (12) compared to controls (10) which was insignificant. But, there was a significant decrease in density of melanocytes in severe dysplasia cases (2) with p=0.003(<0.05) [Table/Fig-5].
Melanocytes, melanin granules and grades of dysplasia.
| Parameters | Normal mucosa(control) | Mild dysplasia(I) | Moderate dysplasia(II) | Severe dysplasia(III) | p-value*, sig | p-value**, sig |
|---|
| Melanocytes (cells/mm) | 10 | 12 | 12 | 2 | 0.003 S | I and III, II and III |
| Melanin granules | 139 | 185 | 223 | 52 | 0.004 S | I and III, II and III |
* Kruskall Wallis
** Mann-Whitney U test
Similar pattern was observed with distribution of melanin granules. There was an increase in melanin granules in mild and moderate dysplasia compared to control which was insignificant. But significant decrease was seen in severe dysplasia with p=0.004 (<0.05) was noted [Table/Fig-5].
Discussion
Oral mucous membrane is composed of stratified squamous epithelium that is supported by fibrous connective tissue (lamina propria and a submucosa of fibroadipose tissue) [4]. The oral mucosal alterations have, for centuries, been intimately involved in the advancement of understanding of potentially malignant disorders, which have an increased likelihood of progressing to oral squamous cell carcinoma [2].
Tobacco usage as both smoking and chewing form is the most common chemical agent responsible for such changes. Aromatic hydrocarbon benz-pyrene and other tar derivatives of tobacco are considered mainly responsible for such alterations [10].
Several physiological and response systems of the body such as, variation in pigmentation and density of melanocyte has been found in relation to chemical agents. The pigment acts as protective barrier against toxins including tobacco [11,5]. No studies have been recorded in literature regarding correlation of melanocytes and melanin pigment with different grades of OED. So this is the pioneer study for this new brainwave.
In the present study, HMB-45 an immunohistochemical stain was used to detect activated melanocytes [12]. HMB-45 is considered to have low false-positive results with greater diagnostic value [13,14] and specificity of 97% [15].
The current study revealed insignificantly increased melanocytes and melanin granules in mild and moderate dysplasia but, significantly reduced in severe epithelial dysplasia [Table/Fig-1].
There are studies which exhibited variations of melanocytes and melanin pigmentation with different type of habit history. Sarswathi TR et al., [16] itemized an increase in number of melanocytes in patients with smoking habit. The increase was due to the chemical irritants like tobacco can cause increase in number of melanocytes [2]. Hedin CA, and Axell T et al., also found clinically visible melanin pigmentation which they believed was due to oral epithelial melanin units acting as defense barrier similar to those in skin. Oral melanocytes thus prevent deleterious effects on cells by binding to polycyclic amines present in tobacco [5]. While severe or long term exposure to chemical irritants can cause death of melanocytes [12].
Long term exposure to tobacco transforms premalignant lesion to malignancy [17]. The severity of dysplasia is proportional to the amount and duration of tobacco habits [18].
Following similar pattern we believe that there could be initial increase in melanocytes in mild dysplasia followed by progressive decrease in number of melanocytes with long term and severe cumulative toxicity of tobacco eventually leading to near or total loss of melanocytes.
Limitation
Scattered details were available regarding detailed history of habits in archives and studies were not recorded in literature regarding role of melanocytes in different grades of OED.
Future scope:
Culturing of oral mucosal melanocytes can be attempted to study the exact mechanism of their activity and destruction on exposure to toxic products.
Conclusion
Based on the results of present study, we conclude that density of melanocytes increases slightly at the stage of mild and moderate dysplasia and decreases significantly as severity of OED increases. The lack of any documentary and supportive evidences related to role of melanocytes with severity of epithelial dysplasia prompted us to carry out this study. The result of present study provides information on variation in melanocytes and melanin pigment in different grades of epithelial dysplasia.
* Kruskall Wallis
** Mann-Whitney U test
[1]. Speight PM, Update on epithelial dysplasia and progression to cancerHead and Neck Pathol 2007 1:61-66. [Google Scholar]
[2]. Bouquot JE, Speight PM, Farthing PM, Epithelial dysplasia of the oral mucosa—diagnostic problems and prognostic featuresCurrent Diagnostic Pathology 2006 12:11-21. [Google Scholar]
[3]. Jaber MA, Porter SR, Gilthorpe MS, Bedi R, Scully C, Risk factors for oral epithelial dysplasia-the role of smoking and alcoholOral oncol 1999 35:151-56. [Google Scholar]
[4]. Greer RO, Pathology of malignant and premalignant oral epithelial lesionsOtolaryngol Clin North Am 2006 39(2):249-75. [Google Scholar]
[5]. Hedin CA, Axell T, Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker’s melanosisJ Oral Pathol Med 1991 20:08-12. [Google Scholar]
[6]. Barrett AW, Scully C, Human oral mucosal melanocytes: a reviewJ Oral Pathol Med 1994 23:97-103. [Google Scholar]
[7]. Ahmed I, Piepkorn M, Godgor DE, Albright LAC, Mayer LJ, Skolnick MH, HMB 45 staining of dysplastic melanocytic nevi in melanoma risk groupsJ Cutan Pathol 1991 18(4):257-60. [Google Scholar]
[8]. World Health Organization Classification of Tumours. Pathology & genetics. Head and neck tumours. International Agency for Research on Cancer (IARC). In: Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Head and neck tumors. Lyon: IARC Press, 2005;177-80 [Google Scholar]
[9]. Patsakas A, Demetriou N, Angelopoulos A, Melanin pigmentation in human gingivaJ periodontal 1981 52(11):701-04. [Google Scholar]
[10]. Slootweg PJ, Thijs A, Merkx W, Premalignant Lesions of the Oral Cavity. In: Barnes L, editorSurgical pathology of Head and Neck 2009 3rd edNew YorkLibrary of congress cataloging-in-publication data:267-84. [Google Scholar]
[11]. Messadi DV, Waibel JS, Mirowski GW, White lesions of the oral cavityDermatol Clin 2003 21:63-78. [Google Scholar]
[12]. Gazit D, Danels TE, Oral melanocytic lesions: differences in expression of HMB-45 and S100 antigens in round and spindle cells of malignant and benign lesionsJ Oral Pathol Med 1994 23:60-64. [Google Scholar]
[13]. Jaber MA, Porter SR, Scully C, Gilthorpe MS, Bedi R, The role of alcohol in non-smokers and tobacco in non-drinkers in the aetiology of oral epithelial dysplasiaInt J Cancer 1998 29;77(3):333-36. [Google Scholar]
[14]. Churukia CJ, Pigments and minerals. In: Bancroft JD, Gamble M, editorsTheory and practice of histological techniques 2002 5th edChurchill LivingstoneElseviere Limited:250-53. [Google Scholar]
[15]. Sheffield MV, Yee H, Dorvault CC, Weilbaecher KN, Eltoum AI, Siegal GP, Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparationsAm J Clin Pathol 2002 118:930-36. [Google Scholar]
[16]. Sarswathi TR, Kumar SN, Kavitha KM, Oral melanin pigmentation in smoked and smokeless tobacco users in India. Clinco pathological studyIndian J Dent Res 2003 14(2):101-06. [Google Scholar]
[17]. Garg KN, Raj V, Chandra S, Trends in frequency and duration of tobacco habit in relation to potentially malignant lesion: A 3 years retrospective studyJ Oral Maxillofac Pathol 2013 17(2):201-06. [Google Scholar]
[18]. Bisht RS, Singh AK, Sikarwar V, Darbari A, Study over the clinical picture and histopathology of leukoplakia and to establish the correlation between causative factors in the patients of Garhwal hill regionNatl J Maxillofac Surg 2013 4(2):177-80. [Google Scholar]