Management of patients with primary IDC breast is based on several clinical and histological prognostic factors. These include age, tumour size, lymph node involvement, histologic type, tumour grade as well as determination of ER, PR and HER2/neu expression. The use of hormone-directed therapies such as Selective Estrogen Receptor Modulators (SERMs) requires accurate determination of status of these hormone receptors. In addition, it has been found that HER2 amplification is an independent negative predictor of overall survival and time to relapse [1].
Studies have also shown beneficial effects of monoclonal antibody trastuzumab (Herceptin) in addition to chemotherapy in early Her2/neu positive breast carcinomas [2]. NAC is used for patients with locally advanced breast carcinoma as well as for patients with metastatic or inoperable breast carcinoma to reduce tumour size and subsequently improve breast-conserving surgery rates [3].
Whether or not NAC affects the receptor status in breast carcinomas is still an unanswered question. Post NAC, a number of changes has been described in the tumour morphology [4]. These can interfere with post NAC grading of the tumour and alter prognostic assessment.
The aim of this study was to compare the IHC profiles of ER, PR and HER2/neu in primary IDC breast before and after NAC to assess the subsequent effects on receptor status. We have also evaluated the differences in morphology of tumour in core biopsies and corresponding post NAC MRM specimens.
Materials and Methods
The present retrospective and prospective study was conducted at Department of Pathology of PGIMER and Dr RML Hospital, New Delhi, India. Eighty-nine female patients with breast carcinoma who underwent needle core biopsy with a diagnosis of IDC, Not Otherwise Specified (NOS) in a period of two years between June 2012 to May 2014 were identified from the database of Department of Pathology. Among these patients, only those who underwent NAC followed by a MRM and a complete surgical pathology report were included in the present study.
NAC regimens included combination of anthracyclines, taxanes, and alkylating agents. Transtuzumab was used for patients with HER2/neu positive core biopsies. Cases of early breast carcinoma in which NAC was not given were excluded as were those who were referred to another institute, were lost to follow up or died before surgery. Cases with incomplete surgical pathology reports of either core biopsy or mastectomy specimen were also excluded. For all the cases included in the study, consent was obtained. Ethical clearance was obtained from Internal Review Board.
Demographic and histologic data was collected, including age, grade, amount of necrosis post NAC and IHC panel for ER, PR and HER2/neu in core biopsies.
All the cases were reviewed and tumours were graded in both pre and post NAC specimens according to Nottingham Histologic Score system (the Elston-Ellis modification of Scarff-Bloom-Richardson grading system). Post chemotherapy changes in the post-NAC MRM specimen were noted. The same IHC panel of ER, PR and HER2/neu was applied on residual tumours in post NAC MRM specimens. The IHC stained slides of both, pre- and post-NAC specimens were evaluated under light microscopy by a single pathologist for uniformity.
For ER and PR scoring, staining of >10% of tumours cells was taken as positive while <10% was taken as negative. For HER2, scoring was done as follows: no staining=0, weak and incomplete membranous staining in tumour cells=1+, moderate, complete membranous staining in at least 10% of invasive tumour cells or intense, incomplete membrane staining in 30% or less of tumour cells=2+, strong, complete membranous staining in more than 30% of tumour cells=3+. IDC with a score of 0 and 1+ was considered negative while those with a score of 2+ and 3+ were considered positive.
The expression of ER, PR and Her2/neu and tumour grades assigned to core biopsies and post NAC MRM specimens were compared. Percent positivity for each receptor was calculated in both pre- and post- chemotherapy cases. Comparison between the two groups was done by McNemar’s test using SPSS software. Significance was defined at p< 0.05.
Results
After fulfillment of exclusion criteria, the final sample size was 32. Patients ranged from 30 years to 75 years (mean=49 years). On histological grading of pre NAC biopsies, 9/32 (28%) were Grade I, 16/32 (50%) were Grade II and 7/32 (22%) were Grade III.
Among the post NAC specimens, 14 (44%) could not be graded due to marked NAC induced changes. Among cases with marked NAC induced changed, 7 (50%) were Grade II and 7 (50%) were Grade III on core biopsy. These changes were not observed in any case with Grade I tumour in core biopsy. Among the MRM tumours that could be graded, 16 cases (50%) retained the same grade while two cases showed a higher grade than the pre NAC biopsy. Five (16%) of the cases showed >10% tumour necrosis.
On immunohistochemical examination, among pre-NAC biopsies, 18 (56%) were ER-positive, 16 (50%) were PR-positive and 10 (31%) were Her-2 neu positive. In Post NAC specimens, 18 (56%) were ER-positive, 7 (22%) were PR-positive and 7 (22%) were Her-2 neu positive. There was no statistically significant difference in ER and Her2/neu expression between Pre- and Post-NAC specimens. However, a statistically significant loss of PR expression was noted between the two groups. [Table/Fig-1] shows percent positivity of ER, PR and Her2/neu in pre- and post-NAC specimens. [Table/Fig-2] shows post-NAC change in ER, PR and Her2 status. Post chemotherapy loss of PR was seen in ten cases and only one case showed gain. [Table/Fig-3] shows a triple positive IDC with post chemotherapy loss of PR.
Comparison of pre- and post-NAC status of ER, PR and Her2/neu in breast carcinomas.
| Marker | Pre NAC positive | Post Nāc positive | p-value |
|---|
| ER (n=32) | 18 (56%) | 18 (56%) | 0.75 |
| PR (n=32) | 16 (50%) | 7 (22%) | 0.04 |
| Her2/neu (n=32) | 10 (31%) | 7 (22%) | 0.51 |
Number of cases showing a change in the ER, PR and Her2/neu status after NAC.
| IHC Marker | Cases showing Post-chemotherapy loss of staining | Cases showing Post-chemotherapy gain of staining |
|---|
| ER | 2 | 2 |
| PR | 10 | 1 |
| Her2/neu | 4 | 1 |
A case of breast carcinoma with ER, PR and Her2/neu Positivity on Pre-NAC Breast core biopsy showed loss of PR positivity on IHC in Post-NAC MRM specimen from the same patient: a-c) Her2/neu, ER and PR respectively in breast core biopsy; d-f) Her2/neu, ER and PR respectively in MRM. Note the loss of PR staining in MRM. (40X)

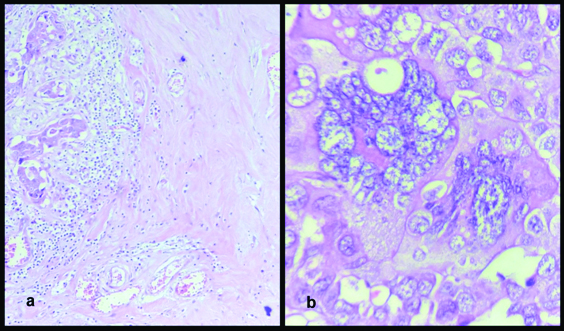
In 44% of cases significant chemotherapy related changes were observed. These changes included cytomorphological changes in malignant cells, most common being dyscohesion, cytoplasmic vacuolization, nuclear vacuolization, multinucleation, large bizzare hyperchromatic nuclei and karyorrhexis. Among stromal changes, extensive collagenization and hyalinization were most common. In two cases, stromal neovascularization was also noted [Table/Fig-4].
a) Extensively collagenised stroma, (H&E, 40X) b) Large bizarre cells with hyperchromatic nuclei and multinucleation, (H&E, 100X).
Discussion
Present day management of Primary IDC include determination of Hormone receptors and Her2 status to assess the utility of hormone-directed therapies such as SERMs and of monoclonal antibody trastuzumab (Herceptin) [1,2]. The prognosis of breast carcinoma is predicted on the basis of several parameters. However, as the treatment options are evolving, a need for change in approach to determine these factors is being felt.
Several authors have noted a change in hormone receptor status after NAC, but without any clear consensus [5-8]. A study by Kinsella MD et al., on 38 patients showed statistically significant loss of PR after NAC while ER and Her2/neu did not show any significant change [5]. Kasami M et al., in their study, found a statistically significant negative change in PR status in 28.8% of patients (n=173) but no significant change in ER was noted after neoadjuvant chemotherapy [6]. Their findings are similar to the present study. In contrast, statistically significant changes in hormone receptor status of primary breast carcinomas following NAC have been reported by Taucher S et al., in a series of 214 patients. According to their study, 14% demonstrated a statistically significant loss of expression of ER in the post treatment final surgical specimen while 51.7% showed a significant loss of PR expression when compared with matched controls [7]. Pedrini JL et al., found weak correlation of Her2/neu and prolactin receptors between pre and post NAC specimens, but no changes in ER or PR receptors [8]. Arens N et al., studied 25 patients and reported no significant differences in expression patterns of ER or PR following neoadjuvant chemotherapy on comparison with matched control patients who did not received neoadjuvant chemotherapy. Though there were rare cases in both groups in which hormone receptor expression changed in the final surgical specimen when compared with the initial core biopsy, these changes were not found to be statistical significance [9].
In our study, statistically significant loss of PR positivity was noticed after NAC. However, ER and Her-2 neu receptors did not show any significant change. This reduction in PR could be in part due to differential tumour sampling between the core biopsy and the final surgical specimen or chemotherapy selective cytotoxicity of PR-expressing cells. According to Pedrini JL et al., clonal selection of tumours cells may occur due to chemotherapy resulting in differences between the IHC expression in pre- and post- NAC samples [8]. They also suggested that different chemotherapeutic agents used in the above studies may account for widely variable results. This hypothesis gains some supported by the observation that the present study as well as the studies by Kinsella MD et al., and Kasami N et al., which used combination chemotherapeutic agents showed loss of PR in post NAC specimens [5,6]. On the other hand, Pedrini JL et al., used anthracyclin based chemotherapy and showed no change in ER and PR [8]. However, these hypotheses have not been verified and further studies are needed. In addition, the manner in which the change in this receptor status affects the treatment of breast carcinoma patients remains to be evaluated in a clinical setting.
Extensive post-NAC changes have been described in breast carcinomas [4,10-13]. These morphological changes may interfere with accurate histological grading of breast carcinomas. Such a grade may not have a prognostic significance [14]. A possible solution is to use the grade assigned to the core biopsies. In the present study, out of 18 post-NAC cases in which grading was possible, 16 (89%) showed the same grade. However, two cases showed a higher grade. This can be explained by much lesser amount of tumour present in biopsy as compared to MRM specimen and increase in cytological atypia after NAC.
Limitation
We did not receive any Invasive Lobular Carcinoma (ILC) during the period of the study and therefore Post NAC changes in ILC cannot be commented upon. The exact mechanism of loss of PR receptors was also outside the scope of this study and requires further investigation.
Conclusion
Since, accurate determination of ER, PR and Her2/neu status in primary IDC breast is important to guide further treatment, changes in receptor status post NAC may warrant corresponding change in hormonal therapy. Reevaluation of ER, PR and Her2/neu status after NAC seems to be required to guide further adjuvant therapy. In addition, post NAC changes in tumour morphology can interfere with grading and its prognostic significance. Therefore, assessment of tumour grade in pre NAC core biopsy itself assumes a greater significance.