Unusual Skin Carcinomas Induced by BRAF Inhibitor for Metastatic Melanoma: A Case Report
Stefano Cavalieri1, Lorenza Di Guardo2, Mara Cossa3, Carolina Cimminiello4, Michele Del Vecchio5
1 Faculty, Department of Medical Oncology, Fondazione Istituto Nazionale dei Tumori, Milan, Italy.
2 Faculty, Department of Medical Oncology, Fondazione Istituto Nazionale dei Tumori, Milan, Italy.
3 Faculty, Department of Pathology, Fondazione Istituto Nazionale dei Tumori, Milan, Italy.
4 Faculty, Department of Medical Oncology, Fondazione Istituto Nazionale dei Tumori, Milan, Italy.
5 Faculty, Department of Medical Oncology, Fondazione Istituto Nazionale dei Tumori, Milan, Italy.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Stefano Cavalieri, via Venezian 1, 20133, Milan, Italy.
E-mail: stefano.cavalieri@istitutotumori.mi.it
The most frequently reported skin tumours during treatment with targeted therapies for BRAF (B type Rapidly Accelerated Fibrosarcoma kinase) mutated metastatic melanoma are squamous cell carcinomas (SCCs). Basal cell carcinomas (BCCs) have been described in such setting, but no cases of multiple and recurring tumours have been reported so far. A patient with a history of chronic sun exposure and more than 10 BCCs removed since 1998 started treatment with vemurafenib for BRAF mutated metastatic melanoma. Therapy was complicated by sporadic episodes of atrial fibrillation and by the development of recurrent, multiple and diffuse BCCs. So, vemurafenib was discontinued and dabrafenib and trametinib were started. Since then, only four BCCs occurred in the patient. Histopathological re-examination showed that most BCCs occurred under vemurafenib presented with squamous features. Such characteristic was significantly less evident before therapy start and in lesions removed under treatment with dabrafenib and trametinib. BRAF inhibition (BRAFi) without MEK inhibition induces mitogen activated kinases overactivation, with consequent skin toxicity and acquired drug resistance. The BCCs removed from our patient showed squamous features, more evident during vemurafenib monotherapy. Both the switch from vemurafenib to dabrafenib and the addition of MEK inhibitor (MEKi) might have reduced the incidence of BCCs and their squamous differentiation.
Basal cell carcinoma, Dabrafenib, Trametinib, Vemurafenib
Case Report
In 2000 a 48-year-old man, with a type II skin according to Fitzpatrick scale [1], was diagnosed with multiple BCCs (at least 10 from 1998) on the trunk and both arms. The patient had a history of chronic sun exposition and in the following years the patient underwent regular dermatological follow up. Thereafter, the medical history was unremarkable until 2008, when the patient was diagnosed with a pigmented lesion on his face (left zygomatic region). Histopathological examination revealed a nodular melanoma, with 1.8 mm Breslow’s thickness, IV Clark’s level, not ulcerated, with 2 mitoses/sqmm (stage pT2a according to seventh edition of American Joint Committee on Cancer) [2].
Surgical wide excision and left neck sentinel lymph node biopsy showed no evidence of disease. Staging exams excluded distant metastases, thus the patient continued clinical follow up.
In 2011, due to occurrence of palpitations, the patient was diagnosed with atrial fibrillation, which resolved after electrical cardioversion. In the same period, a chest radiograph showed a nodule in right lung, which was confirmed by Computed Tomography (CT) scan and by Positron Emission Tomography (PET). Such whole body staging exams did not reveal any other disease localizations.
In October 2011, a segmentectomy of basal lateral right lung was performed by video assisted thoracoscopy. Histological examination revealed a lung metastasis from melanoma and molecular analysis showed V600E mutation in exon 15 of BRAF gene. Because of the absence of other distant lesions, the patient underwent follow up, but eight months after surgery (June 2012) a wb CT scan revealed disease progression in lung.
Due to disease stage, to short disease free interval and to BRAF mutation in July 2012, biologic treatment with vemurafenib 1920 mg daily was started. A prompt clinical response was revealed by disease re-assessments, but six months after start of treatment, a new episode of atrial fibrillation occurred, which was treated with pharmacological cardioversion and anticoagulant therapy. Such treatment has been continued until now and no further cardiac adverse events have been observed since then.
Clinical response was maintained in the following months (wb CT scans persistently negative till February 2014), but multiple and diffuse BCCs started occurring after one year from start of treatment (July 2013). A total of 18 BCCs were removed from several anatomic areas (trunk and both arms and legs) [Table/Fig-1] One actinic keratosis and one low grade (G1) SCC appeared on sun exposed skin as well. Due to the recurrence of BCCs and the increasing photosensitivity, the treatment with vemurafenib was discontinued after 28 months. Therefore, in October 2014 treatment with dabrafenib 300 mg daily and trametinib 2 mg daily was started. Baseline echocardiogram, cardiological and ophthalmological evaluations were unremarkable.
Macroscopic findings of multiple and diffuse BCCs.
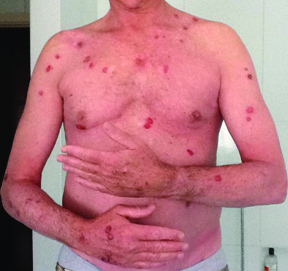
Combination therapy with BRAFi and MEKi was well tolerated. During such treatment a new primitive superficial pT2a melanoma appeared on the skin of right temporal region. Nonetheless, the combined targeted therapy was continued, due to good response to treatment.
Since dabrafenib and trametinib treatment start, a total of four BCCs were surgically excised. No further significant adverse events have been observed and a clinical response is maintained to date. No dose reductions were performed.
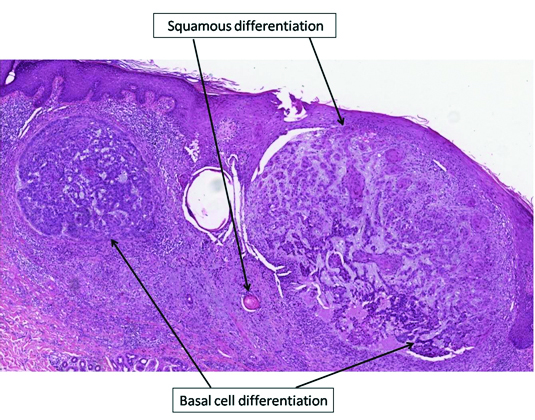
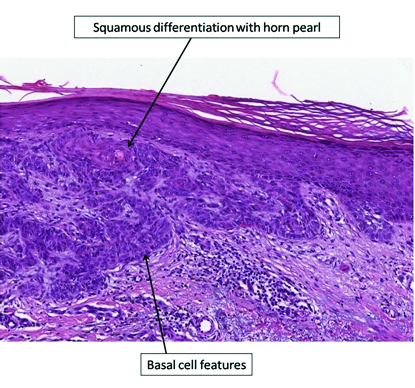
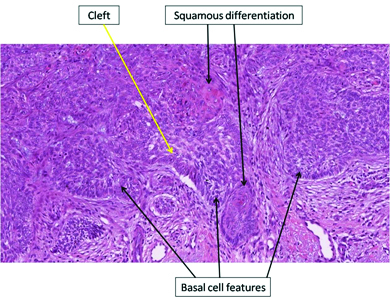
The histo-pathology of all the BCC specimens was retrospectively analysed in order to evaluate any morphological differences between the various skin lesions [Table/Fig-2]. Differently from the pre-treatment period, when BCCs presented a classical morphology, during therapy with vemurafenib a squamous differentiation was observed in most tumours [Table/Fig-3,4,5]. The number of BCCs and their squamous differentiation have significantly reduced since dabrafenib and trametinib start. In the period without treatment, 63% (5/8) of BCCs had a classical morphology and only three had focal features of squamous differentiation. Under vemurafenib, squamous characteristics were observed in 83% (15/18) of cases, whereas only two (11%) had a classical morphology; one actinic keratosis and one SCC of the skin were observed as well. Such lesions have not been reported in the period without treatment. During combined therapy with dabrafenib and trametinib, only one BCC presented with focal features of squamous differentiation, whereas the remaining three BCCs had a classical morphology.
Histopathological features of patient’s BCCs.
| No treatment | Vemurafenib | Dabrafenib + trametinib |
|---|
| BCCs | 8 | 18 | 4 |
| MorphologyClassicalSquamous differentiationOther | 3 (37%)3 (37%)2 (25%) | 2 (11%)15 (83%)1 (6%) | 3 (75%)1 (25%)0 |
| InfiltrationAbsent (superficial BCC)Papillary dermisReticular dermisHypodermis | 2 (25%)05 (62%)1 (13%) | 1 (6%)8 (44%)8 (44%)1 (6%) | 04 (100%)00 |
| UlcerationAbsentPresent | 8 (100%)0 | 14 (78%)4 (22%) | 4 (100%)0 |
| InflammationMildModerateIntense | 3 (37%)2 (25%)3 (37%) | 3 (17%)6 (33%)9 (50%) | 04 (100%)0 |
Histological findings of BCCs with squamous features (haematoxylin and eosin stain, 4X magnification).
Histological findings of BCCs with squamous features (haematoxylin and eosin stain, 10X magnification).
Histological findings of BCCs with squamous features (haematoxylin and eosin stain, 10X magnification).
Discussion
Approximately 50% of metastatic melanomas are driven by mutations in BRAF gene and, in these cases, specific targeted inhibitors demonstrated to have an impressive anti tumour efficacy also in terms of very rapid responses even on large tumour burden. These drugs are generally well tolerated; skin toxicity and fever with arthralgias represent the most frequent adverse events reported with vemurafenib and dabrafenib monotherapy, respectively [3]. The recent introduction of MEKi, such as cobimetinib and trametinib, allowed to meet three important endpoints: increase of the response rate observed with BRAF inhibitor-based mono-targeted therapy (69% versus 53%) [4-6];prolongation of the median response duration interfering with some mechanisms of resistance acting on the Mitogen-Activated Protein Kinase (MAPK) signalling pathway; improvement of the cutaneous toxicity profile, especially in terms of reduction in the incidence of SCC (SCCs - from 11% to 3%). Nevertheless, up to 3% of subjects treated with the combined approach is diagnosed with non melanoma skin cancers during treatment [4,5]. BCCs with BRAFi have been described so far, but no cases of multiple and recurring tumours have ever been reported. The majority of BCCs occur in the head and neck region, followed by trunk and extremities. This anatomical distribution reflects the pathogenetic role of sun exposure [7], and it was seen in our case too.
The development of non melanoma skin cancers like SCCs and keratoacanthomas in patients under treatment with BRAFi is driven by paradoxical overactivation of the MAPK signaling pathway in epithelial cells harboring RAS mutation [8]. This gene encodes for a GTPase activating RAF kinase, that in turns activates MEK, a mitogen-activated kinase. Consequently, the association of MEKi with BRAFi leads to an impressive reduction of SCC incidence. [9]. Furthermore, SCC pathogenesis has been recently associated with BRAF-CRAF heterodimerization [10]. Combo-targeted therapies with BRAFi and MEKi improve both overall and progression-free survivals when compared to BRAFi alone [4-6]. Such improvement is also associated with a reduction in skin toxicity. In the Combi-V trial, the occurrence of SCC and keratoacanthoma was described in 1% of patients in the combination-therapy group and in 18% of those in the vemurafenib one. [5]. A possible mechanism explaining what we observed could be the involvement of Sonic Hedgehog Homolog (Shh) pathway. Shh can be over-activated by BRAFi and it is known to play a role in the development of drug resistance mediated by secondary PDGFRα up-regulation [11]. Shh pathway plays a pathogenetic role in BCC development [12] and its inhibition is the biological basis of systemic treatment of advanced BCC [13].
The addition of MEKi to BRAFi significantly reduces the incidence of non melanoma skin cancers. Likely, subjects intrinsically predisposed to the development of such tumours, like our patient, may suffer more intensively from BRAFi induced skin toxicity. Our case taught us that vemurafenib might induce two main phenomena: The numeric growth of BCCs and their squamous differentiation.
Conclusion
BCCs in patients under treatment with BRAF inhibitors have been described in the scientific literature, but no cases of multiple and recurring BCCs have ever been reported in this setting. Our patient had an intrinsic susceptibility in developing BCCs, since a considerable number of them occurred without any treatments. After vemurafenib discontinuation and combined therapy start, a substantial modification in quality and quantity of BCCs has been observed. The number of such lesions significantly decreased from 18 BCCs in 28 months to four BCCs in 20 months, from a mean of 1 BCC every 1.5 months with vemurafenib to a mean of 1 BCC every five months with dabrafenib and trametinib.
[1]. Roberts WE, Skin type classification systems old and newDermatologic clinics 2009 1027(4):529-33. [Google Scholar]
[2]. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Final version of 2009 AJCC melanoma staging and classificationJournal of clinical oncology :Official journal of the American Society of Clinical Oncology 2009 Dec2027(36):6199-206. [Google Scholar]
[3]. Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trialLancet (London, England) 2012 Jul28380(9839):358-65. [Google Scholar]
[4]. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trialLancet (London, England) 2015 Aug01386(9992):444-51. [Google Scholar]
[5]. Grob JJ, Amonkar MM, Karaszewska B, Schachter J, Dummer R, Mackiewicz A, Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v):Results of a phase 3, open-label, randomised trialThe Lancet Oncology 2015 1016(13):1389-98. [Google Scholar]
[6]. Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Combined vemurafenib and cobimetinib in BRAF-mutated melanomaThe New England journal of medicine 2014 Nov13371(20):1867-76. [Google Scholar]
[7]. Verkouteren JA, Ramdas KH, Wakkee M, Nijsten T, Epidemiology of basal cell carcinoma: Scholarly reviewBr J Dermatol 2017 0220Epub ahead of print [Google Scholar]
[8]. Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitorsThe New England journal of medicine 2012 Jan19366(3):207-15. [Google Scholar]
[9]. Carlos G, Anforth R, Clements A, Menzies AM, Carlino MS, Chou S, Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination With MEK Inhibitors for Metastatic MelanomaJAMA dermatology 2015 10151(10):1103-09. [Google Scholar]
[10]. Boussemart L, Girault I, Malka-Mahieu H, Mateus C, Routier E, Rubington M, Secondary Tumors Arising in Patients Undergoing BRAF Inhibitor Therapy Exhibit Increased BRAF-CRAF HeterodimerizationCancer research 2016 Mar1576(6):1476-84. [Google Scholar]
[11]. Sabbatino F, Wang Y, Wang X, Flaherty KT, Yu L, Pepin D, PDGF Ralpha up-regulation mediated by sonic hedgehog pathway activation leads to BRAF inhibitor resistance in melanoma cells with BRAF mutationOncotarget 2014 04155(7):1926-41. [Google Scholar]
[12]. Dahmane N, Lee J, Robins P, Heller P, Ruizi Altaba A, Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumoursNature 1997 Oct23389(6653):876-81. [Google Scholar]
[13]. Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Efficacy and safety of vismodegib in advanced basal-cell carcinomaThe New England journal of medicine 2012 Jun07366(23):2171-79. [Google Scholar]