Introduction
Insulin resistance is a state of reduced response of peripheral tissues to insulin, characterized by hyperinsulinemia, enhanced lipolysis and pronounced accumulation of fat in peripheral tissues [1]. It contributes to the development of NAFLD and AS. NAFLD is a chronic liver disorder worldwide, commonly associated with metabolic syndrome including Type 2 Diabetes Mellitus (T2DM), dyslipidemia, AS, hypertension etc., [2]. It is characterized by HS which can progress to cirrhosis and hepatocellular carcinoma [3]. There is an increased incidence of cardiovascular disease related morbidity and mortality associated with NAFLD and AS which suggests the importance of early interventions to treat this syndrome[4].
IR may play a vital role in the development of AS [5]. AS is an inflammatory disease characterized by the accumulation of fat in the inner wall of arteries. It results in narrowing and hardening of the arteries and proliferation of intimal-smooth muscle cells leading to development of atheromatous plaques [6]. NAFLD is strongly accompanied with AS which results in cardiovascular abnormalities. It is primarily associated with dyslipidemia shows an elevation in hepatic triglycerides which alters the atherogenic lipid levels i.e., decreased HDL cholesterol and raised LDL cholesterol. In addition to these changes, raised Free Fatty Acids (FFAs) can also impair insulin levels which contribute to AS [7].
Large doses or long term Glucocorticoids (GCs) therapy can produce IR, hyperinsulinemia and cardiovascular disorders [8]. GCs induced IR may trigger the fat accumulation in liver and arteries. It can decrease hepatic insulin sensitivity [9,10].
PIO, a Thiazolidinedione (TZD), act through the stimulation of Peroxisome Proliferator Activated Receptor (PPAR)-ϒ and inturn regulates the insulin signaling pathway. It enhances insulin sensitivity in liver and peripheral tissues and maintains the carbohydrate and lipid metabolism [11]. MET, a biguanide, activates the enzyme Adenosine Monophosphate Kinase (AMPK) in the liver. It inhibits hepatic gluconeogenesis, enhances glucose uptake in peripheral tissues and decreases glucose absorption from intestine [12].
It has been approved that both PIO and MET drugs are effective insulin sensitizers in the treatment of T2DM [13,14]. PIO appeared to prevent endothelial dysfunction in diabetes mellitus [15]. It has been shown to decrease the generation of superoxide radical and downregulate the expression of the LOX-1 gene and monocyte adhesion to endothelium [16]. In addition, it reduces the expression of vascular cell adhesion molecule 1(VCAM-1) subsequently inhibits the formation of atheromatous plaque in endothelium. These actions are mediated by PPAR-ϒ activation [17]. MET also improves endothelial function by decreasing oxidative stress through opposing the NADPH oxidase and Protein Kinase C (PKC) pathways. It can inhibit smooth muscle cell proliferation and prevents the plaque formation [18].
TZD like PIO has a therapeutic role in NAFLD. It may act by redistributing fat from liver to adipose tissue. It reduces the incidence of HS and consequent hepatocellular injury in T2DM. It is effective in improving biochemical and histological abnormalities in NAFLD which explain its efficacy [19]. MET is also a promising drug in the treatment of NAFLD. It regulates the lipid metabolism and reduces the fat content in liver through its AMPK activation [20]. It has been shown to be effective in controlling biochemical and metabolic alterations of NAFLD [21]. But contradictory findings have been observed in view of histological improvement in NAFLD [22].
Both PIO and MET drugs are well known insulin sensitizers selected for the present study. Recent studies have compared the efficacy of PIO and MET in the treatment of T2DM [23,24]. However, there is a scarcity of data to explain the relative efficacy of MET in comparison to PIO in diabetes associated AS and HS complications. Our study hypothesized that insulin sensitizers may help to reduce the occurrence of AS and HS associated with T2DM. Therefore, we aimed to compare the efficacy of MET and PIO drugs in GCs induced AS and HS in Wistar rats.
Materials and Methods
The animal study was conducted during the month of November, 2015, at KS Hegde Medical Academy, Mangalore, Karnataka, India.
Experimental animals: Around 24 male Wistar albino rats (250-280 gm) were obtained for this study. The study was approved and permission was taken from the Institutional Animal Ethical Committee (IAEC) (KSHEMA/IAEC/02/2013). As per the Committee for Purpose of Control and Supervision on Experimental Animals (CPCSEA), animals were maintained at 23 ± 2ºC temperature, humidity 50 ± 5 %, 12 hours light and 12 hours dark cycles. They were accommodated in polypropylene cages (UN shah manufacturers, Mumbai) and provided pellet food (Hindustan lever limited, Mumbai) and water ad libitum.
Drugs and chemicals: MET and PIO drugs were obtained from Mahalakshmi chemicals, Hyderabad, Telangana state, India. DEX injection was procured from Zydus pharmaceuticals, Mumbai. Ketamine injection was received from Neom Laboratories Limited, Mumbai. Serum insulin levels were estimated by ultrasensitive rat insulin Enzyme-Linked Immuno Sorbent Assay (ELISA) kit, purchased from the Gen X Bio Health Sciences Private Limited, New Delhi. Commercial analytical kits were procured for the estimation of glucose and lipid levels.
Experimental procedure: Animals were divided into four groups (n=6). Group 1 act as normal control which received 2% gum acacia orally for 12 days. Group 2 served as DEX control, treated with 2% gum acacia orally for 12 days and DEX (8 mg/kg/i.p.) from 7th to 12th day during the study period. Group 3 treated as PIO control, received PIO at a dose of 45 mg/kg orally and Group 4 considered as MET control, received MET (1000 mg/kg) orally for 12 days. Both Group 3 and Group 4 rats were treated with DEX (8 mg/kg/i.p.) from 7th to 12th day during the study period. At the end of the study period, fasting blood was collected through the retro-orbital sinus puncture and sacrificed by cervical dislocation under ketamine (50 mg/kg/i.p.) anaesthesia. The blood samples were centrifuged at 2000 RPM for 20 minutes. Serum was separated and used for estimation of glucose, insulin and lipid levels. Liver and aorta tissues were dissected out and stored for further histopathological investigations. Liver weight and liver volume was measured. Body weight of animals was recorded on 1st day and 12th day during the study period.
Histopathology examination: Isolated liver and aorta tissues were fixed in 10% formalin solution. Tissues were sliced to 5 μm thick by using microtome and sections were stained with H&E stain. Further these sections were analysed for fatty changes under the microscope (40X) [25].
Measurement of physical parameters: Body weight and liver weight of rats were measured by using an electrical balance machine. Liver volume was calculated as volume of saline displaced with liver in 100 ml of saline [26].
Estimation of biochemical parameters: The ELISA method was employed to measure the serum insulin levels by using an ELISA reader. Glucose Oxidase and Peroxidase (GOD-POD) method was used to estimate serum glucose levels. Serum Total Cholesterol (TC), High Density Lipoproteins (HDL) and Low Density Lipoproteins (LDL) cholesterol levels were analysed by cholesterol oxidase peroxidase (CHOD-POD) method. Serum Triglyceride (TG) levels were measured by Glycerol 3-Phosphate Oxidase Phenol Aminophenazine (GPO-PAP) method [27].
Calculation of homeostatic model assessment–Insulin resistance (HOMA-IR) [28]: Fasting insulin(µU/ml)×Fasting glucose (mg/dl)/405.
Statistical Analysis
The values were expressed as mean±SEM. The data was analysed by using SPSS statistics 20.0 software version. The results were compared by using One-way Analysis of variance (ANOVA) followed by Scheffe’s multiple comparison post-hoc test. The significance level was fixed at p<0.05.
Results
Effect of standard drugs on histopathology of liver: Group 1 rat liver showed a normal hepatic architecture with round regular nucleus. The cytoplasm was pink and vesicular [Table/Fig-1a]. But treatment with DEX (8 mg/kg/i.p.) altered the hepatic architecture. It showed an increase in the size of hepatocytes and nucleus was pushed to the periphery. The cytoplasm showed large fatty vacuoles [Table/Fig-1b].
a) Control rat liver showed normal hepatocytes: Centrally placed nucleus: cytoplasm is pink and vesicular. (H&E, 40X); b) Dexamethasone treated rat liver showed loss of liver architecture; Hepatocytes showd fatty vacuoles in cytoplasm; Nucleus is pushed to periphery. (H&E, 40X); c) Pioglitazone treated rat liver exhibited an improvement in architecture; Restored fatty changes induced by dexamethasone. (H&E, 40X); d) Metformin treated rat liver showed partial improvement in hepatic parenchyma; Mild fatty changes are seen in cytoplasm. (H&E, 40X)
*Arrow indicates fat deposits
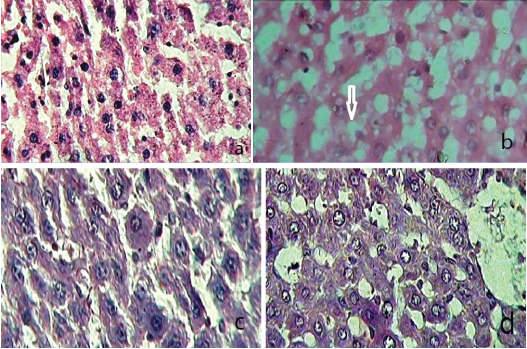
As shown in [Table/Fig-1c,d], Oral administration of PIO (45 mg/ kg) and MET (1000 mg/kg) resulted in complete restoration of DEX induced hepatic fatty changes and improved liver parenchyma. However, PIO treatment seems to be highly effective compared to MET.
Effect of standard drugs on histopathology of aorta: The control rat aorta showed normal tunica intima, media and adventitia layers [Table/Fig-2a]. Intraperitoneal administration of DEX (8 mg/kg) to albino rats showed plump endothelial cells in tunica intima. It caused fat deposition in the cytoplasm and showed small regular nucleus. Nevertheless, no fatty changes found in media and adventitia layers [Table/Fig-2b].
a) Normal control aorta tunica intima, media and adventitia layers. (H&E, 40X); b) Dexamethasone treated rat aorta showed plump endothelial cells in tunica intima; Increased amount of fatty streaks observed in cytoplasm. (H&E, 40X); c) Pioglitazone treated rat aorta exhibited a marked improvement in fatty changes induced by dexamethasone. (H&E, 40X); d) Metformin treated rat aorta showed a decrease in fatty changes induced by dexamethasone; Minimal fatty deposits observed in endothelium. (H&E, 40X)
*Arrow indicates fat deposits
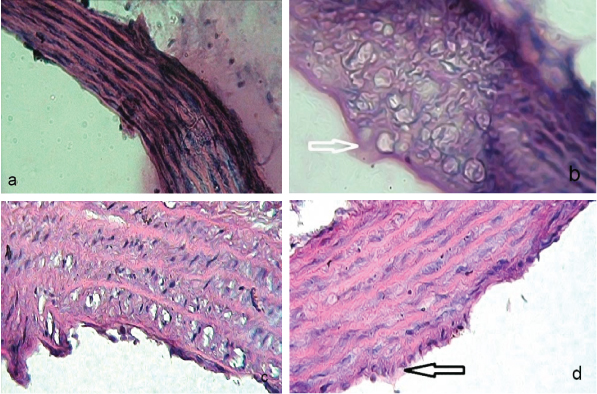
Treatment with PIO and MET had effectively prevented fatty changes in aorta induced by DEX. Overall, PIO appeared to be more effective compared to MET in preventing DEX initiated fatty changes in aorta [Table/Fig-2c,d].
Effect of standard drugs on serum insulin, glucose levels and HOMA-IR as showed in the [Table/Fig-3], intraperitoneal administration of DEX significantly (p<0.05) elevated the serum insulin and glucose levels compared to control rats. It significantly (p<0.05) diminished the insulin sensitivity evidenced by raised HOMA-IR levels. Treatment with PIO and MET significantly (p<0.05) prevented the DEX induced elevation in insulin and glucose levels and inturn improved HOMA-IR in Wistar rats. But, PIO was observed to be highly significant compared to MET drug in improving insulin sensitivity (p<0.05).
Comparison of effect of pioglitazone and metformin on serum glucose, insulin and HOMA-IR levels against dexamethasone induced Insulin resistance (n=6) expressed in Mean±SEM.
| Groups | glucose levels (mg/dl) | Insulin levels (μU/ml) | HÜMA-IR |
|---|
| Group 1 | 99.41±1.23 | 72.48±1.19 | 17.79±0.37 |
| Group 2 | 259.07±3.24*a | 459.24±12.04*a | 293.47±6.00*a |
| Group 3 | 144.75±4.90†bd | 160.76±3.24†bd | 57.59±2.91†bd |
| Group 4 | 168.80±4.36†bc | 219.24±4.88†bc | 91.43±3.42†bc |
p<0.05 compared to Normal control (Group 1);
p<0.05 compared to Dexamethasone control (Group 2);
p<0.05 compared to Pioglitazone control (Group 3);
p<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
Effect of standard drugs on lipid levels as shown in the [Table/Fig-4], treatment with DEX significantly (p<0.05) raised lipid levels i.e., TC, TG, LDL and reduced HDL cholesterol in Wistar rats. However, oral administration of both PIO and MET significantly (p<0.05) reversed the elevated lipid levels in DEX treated rats. A significant difference was observed in between PIO and MET treated group in which PIO treatment has showed more significant improvement in lipid levels (p<0.05).
Comparison of effect of pioglitazone and metformin on serum lipid levels against dexamethasone induced Insulin resistance (n=6) expressed in Mean±SEM.
| Groups | TC (mg/dl) | TG (mg/dl) | HDL Cholesterol (mg/dl) | LDL Cholesterol (mg/dl) |
|---|
| Group 1 | 90.12±4.33 | 65.98±2.36 | 22.42±0.99 | 15.59±0.56 |
| Group 2 | 209.55±4.66*a | 190.38±3.13*a | 9.99±0.33*a | 111.33±1.98*a |
| Group 3 | 105.79±2.37†bd | 89.42±1.98†bd | 21.02±0.48†bd | 49.36±2.61†bd |
| Group 4 | 135.67±2.39†bc | 124.87±14.69†bc | 18.06±0.6†bc | 60.13±1.70†bc |
p<0.05 compared to Normal control(Group 1);
p<0.05 compared to Dexamethasone control (Group 2);
p<0.05 compared to Pioglitazone control (Group 3);
p<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
Effect of PIO and MET on body weight, liver weight and liver volume as detailed in [Table/Fig-5], a significant (p<0.05) decrease in body weight and increase in liver weight and liver volume observed in rats treated with DEX. These DEX induced alterations were significantly (p<0.05) prevented by PIO and MET therapy. However, our study showed that the PIO treatment was highly significant compared to MET in controlling DEX induced changes in rats (p<0.05).
Comparison of effect of pioglitazone and metformin on body weight, liver weight and liver volume against dexamethasone induced Insulin resistance (n=6) expressed in Mean±SEM.
| Groups | Body weight (gm) | Liver weight (gm) | Liver volume (ml) |
|---|
| Day 1 | Day 12 | Day 1 | Day 12 | Day 1 | Day 12 |
|---|
| Group 1 | 250.83± 1.38 | 262.17± 1.3 | 3.14±0.57 | 3.62± 0.13 | 2.0±0.25 | 2.6±0.09 |
| Group 2 | 272.38± 1.42 | 185± 2.86*a | 3.42±1.25 | 13.28± 0.38*a | 2.4±0.47 | 15.36± 0.59*a |
| Group 3 | 263.5± 3.69 | 235.17± 1.51†bd | 3.63±0.39 | 5.21± 0.26†bd | 2.8±0.83 | 4.4± 0.23†bd |
| Group 4 | 257.68± 2.46 | 210.54± 2.63†bc | 3.26±0.48 | 7.48± 1.54†bc | 3.0±0.14 | 6.17± 0.52†bc |
p<0.05 compared to Normal control (Group 1);
p<0.05 compared to Dexamethasone control (Group 2);
p<0.05 compared to Pioglitazone control (Group 3);
p<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
Discussion
GCs are well known anti-inflammatory drugs but the administration of large doses being involved in the development of IR associated complications [29]. DEX can impair insulin signaling in peripheral tissues and causes metabolic alterations leading to IR [30]. Our study also supported the same that the intraperitoneal administration of DEX (8 mg/kg) induced IR evidenced by a significant hyperinsulinemia, hyperglycemia and hyperlipidemia in rats. Further it also showed a marked lipid deposition in the aorta and liver. These findings of the present study indicate the development of AS, HS and IR in albino rats.
The primary risk factors for the generation of AS are hyperinsulinemia and hyperlipidemia [31]. According to Laakso M et al., elevated insulin concentration can promote VLDL synthesis inturn hypertriglyceridemia and also enhances the formation of LDL cholesterol in the vessel wall. These atherogenic lipid forms inturn involved in the development of lipid plaques in the arterial wall. These fatty changes obstruct the normal blood flow in blood vessels resulting in cardiovascular abnormalities [32]. In accordance with previous research findings, our study also exhibited that DEX administration caused a significant elevation in insulin and lipid levels which may contribute to development of AS. Furthermore, histopathological examination of DEX treated rat aorta exhibited a moderate amount of fatty deposits in the intima layer revealed a state of AS. However, our study with PIO and MET monotherapy effectively reduced the DEX induced fatty changes in aorta. In earlier studies, both PIO and MET drugs appeared to decrease the formation of lipid plaques by inhibiting lipid peroxidation and adhesion molecule expression in arterial wall [33,34]. In another study, PIO and MET treatment showed a significant decrease in atherogenic form of LDL cholesterol levels which has ability to infiltrate into the endothelium [35]. In addition to that, PIO and MET drugs can increase the secretion of adiponectin from adipose tissue which has direct preventive effect on AS and IR [36,37]. Overall, the anti-atherosclerotic action of PIO and MET might be attributed to their insulin sensitizing property evidenced by decreased insulin, lipid, glucose and HOMA-IR levels in DEX treated rats. Furthermore, PIO treatment completely restored the fatty changes in aorta whereas MET showed partial improvement in albino rats.
In the current study, treatment with PIO and MET for 12 days completely restored DEX induced fatty changes in the liver. Histopathological examination of rat liver revealed that both the drugs effectively reduced fat deposition in hepatocytes and normalized the liver parenchyma. These actions were further supported by a significant reduction in liver weight and liver volume with PIO and MET therapy. Although these two drugs have anti-steatotic properties, but have distinct mechanism of action [38]. Previous studies established their mechanism of action in which PIO appeared to act as insulin sensitizer through activating PPAR-ϒ and promote movement of lipids from the liver to adipose tissue [39]. MET acts by stimulating AMPK which controls lipid and glucose metabolism. It results in decrease in activity of acetyl CoA carboxylase (ACC) and induces fatty acid oxidation thereby suppress the lipogenic enzymes [40]. The lipid lowering action in liver and insulin sensitizing property may contribute to anti-steatotic activity of PIO and MET. Furthermore, these findings suggest that PIO has highly significant activities compared to MET against HS in albino rats.
In the current study, PIO therapy was associated with a significant increase in body weight, possibly due to lipid accumulation and water retention [41]. Although MET can reduce body weight due to loss of adipose tissue, but DEX induced weight loss significantly prevented by MET [42]. However, PIO treatment was observed to be more effective in terms of weight gain compared to MET.
PIO has been shown to be effective in the treatment of diabetic consequences such as AS and HS [43,44]. But, there was only limited data available in relation to MET efficacy on AS and HS associated with T2DM. In this background, our study was designed to explore the beneficial effects of MET and compare the same with PIO monotherapy to establish its efficacy on DEX induced diabetic complications. In the present study, we demonstrated the quantitative analysis of efficacy of PIO monotherapy revealed a significant reduction in serum insulin levels (77%), glucose levels (72%), TC levels (87%), TG levels (81%), LDL cholesterol levels (65%) and elevation of HDL cholesterol levels (89%) in DEX treated rats. Furthermore, MET monotherapy reduced serum insulin levels (60%), glucose levels (57%), TC levels (62%), TG levels (53%), LDL cholesterol levels (54%) and increased HDL cholesterol levels (65%) in DEX treated rats. DEX induced pathological changes such as AS and HS completely reduced by PIO and partial improvement was observed with MET therapy. Taken together, our study revealed that the PIO was significantly more effective compared to MET in reversing DEX induced complications i.e., AS, HS and IR in albino rats. The insulin sensitizing and hypolipidemic activities of these drugs could be a possible mechanism related to their beneficial effects in AS and HS in albino rats [45,46].
The incidence of GCs induced diabetic complications will continue to rise because of their extensive use to obtain their attractive therapeutic benefits in clinical settings [47]. Hence, there is a need for efficacious insulin sensitizer to counteract the GCs induced complications. In this study, both drugs have been shown to be effective in the treatment of GCs induced diabetic complications. Earlier studies have compared the efficacy of PIO and MET in fatty liver and endothelial disturbances in which no significant differences were observed [48,49]. But, our study clearly pointed out the superiority of PIO monotherapy over MET treatment. In this view, our study suggest that PIO can be a more effective insulin sensitizer compared to MET in ameliorating the diabetic complications associated with DEX therapy.
Limitation
The Special fat stains would be more useful in studying the DEX induced fatty changes in liver and aorta which is a main limitation of the present study. Further comprehensive studies are required to elucidate the exact mechanism of PIO and MET effects in AS and HS.
Conclusion
Our study strongly established the fact that the large doses of DEX can induce IR and inturn contributes to development of AS and HS. It was concluded that the PIO therapy was more effective compared to MET in controlling GCs induced AS, HS and IR in albino rats. However, adverse effects of PIO limiting its utility whereas MET have better tolerability compared to PIO.
*ap<0.05 compared to Normal control (Group 1);
†bp<0.05 compared to Dexamethasone control (Group 2);
†cp<0.05 compared to Pioglitazone control (Group 3);
†dp<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
*ap<0.05 compared to Normal control(Group 1);
†bp<0.05 compared to Dexamethasone control (Group 2);
†cp<0.05 compared to Pioglitazone control (Group 3);
†dp<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
*ap<0.05 compared to Normal control (Group 1);
†bp<0.05 compared to Dexamethasone control (Group 2);
†cp<0.05 compared to Pioglitazone control (Group 3);
†dp<0.05 compared to Metformin group (Group 4) (One-way ANOVA followed by Scheffe’s multiple comparison test)
[1]. Wilcox G, Insulin and insulin resistanceClin Biochem Rev 2005 26(2):19-39. [Google Scholar]
[2]. Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A, Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart diseaseNutrients 2013 5(5):1544-60. [Google Scholar]
[3]. Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G, Cardiovascular and systemic risk in nonalcoholic fatty liver disease-atherosclerosis as a major player in the natural course of NAFLDCurr Pharm Des 2013 19(29):5177-92. [Google Scholar]
[4]. Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z, Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysisSci Rep 2016 6:33386 [Google Scholar]
[5]. Semenkovich CF, Insulin resistance and atherosclerosisJ Clin Invest 2006 116(7):1813-22. [Google Scholar]
[6]. Ross R, Atherosclerosis—an inflammatory diseaseN Engl J Med 1999 340(2):115-26. [Google Scholar]
[7]. Xu X, Lu L, Dong Q, Li X, Zhang N, Xin Y, Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosisLipids Health Dis 2015 14(1):158 [Google Scholar]
[8]. Rafacho A, Marroqui L, Taboga SR, Abrantes JL, Silveira LR, Boschero AC, Glucocorticoids in vivo induce both insulin hypersecretion and enhanced glucose sensitivity of stimulus-secretion coupling in isolated rat isletsEndocrinol 2010 151(1):85-95. [Google Scholar]
[9]. Nagle CA, Klett EL, Coleman RA, Hepatic triacylglycerol accumulation and insulin resistanceJ Lipid Res 2009 50:S74-79. [Google Scholar]
[10]. Chen X, Iqbal N, Boden G, The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjectsJ Clin Invest 1999 103(3):365-72. [Google Scholar]
[11]. Gad MZ, Ehssan NA, Ghiet MH, Wahman LF, Pioglitazone versus metformin in two rat models of glucose intolerance and diabetesPak J Pharm Sci 2010 23(3):305-12. [Google Scholar]
[12]. Nasri H, Rafieian-Kopaei M, Metformin: Current knowledgeJ Res Med Sci 2014 19(7):658-64. [Google Scholar]
[13]. Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and-independent pathwaysJ Biol Chem 2006 281(13):8748-55. [Google Scholar]
[14]. DeFronzo RA, Goodman AM, Efficacy of metformin in patients with non-insulin-dependent diabetes mellitusN Engl J Med 1995 333(9):541-49. [Google Scholar]
[15]. Martens FM, Visseren FL, de Koning EJ, Rabelink TJ, Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetesJ Cardiovasc Pharmacol 2005 46(6):773-78. [Google Scholar]
[16]. Mehta JL, Hu B, Chen J, Li D, Pioglitazone inhibits LOX-1 expression in human coronary artery endothelial cells by reducing intracellular superoxide radical generationArterioscler Thromb Vasc Biol 2003 23(12):2203-08. [Google Scholar]
[17]. Imamoto E, Yoshida N, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cellsBiofactors 2004 20(1):37-47. [Google Scholar]
[18]. Molavi B, Rassouli N, Bagwe S, Rasouli N, A review of thiazolidinediones and metformin in the treatment of type 2 diabetes with focus on cardiovascular complicationsVasc Health Risk Manag 2007 3(6):967-73. [Google Scholar]
[19]. Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, A pilot study of pioglitazone treatment for nonalcoholic steatohepatitisHepatology 2004 39(1):188-96. [Google Scholar]
[20]. Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Metformin versus dietary treatment in nonalcoholic hepatic steatosis: A randomized studyInt J Obes 2010 34(8):1255-64. [Google Scholar]
[21]. Rouabhia S, Milic N, Abenavoli L, Metformin in the treatment of non-alcoholic fatty liver disease: Safety, efficacy and mechanismExpert Rev Gastroenterol Hepatol 2014 8(4):343-49. [Google Scholar]
[22]. Li Y, Liu L, Wang B, Wang J, Chen D, Metformin in non-alcoholic fatty liver disease: A systematic review and meta analysisBiomed Rep 2013 1(1):57-64. [Google Scholar]
[23]. Pavo I, Jermendy G, Varkonyi TT, Kerenyi Z, Gyimesi A, Shoustov S, Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetesJ Clin Endocrinol Metab 2003 88(4):1637-45. [Google Scholar]
[24]. Yamanouchi T, Sakai T, Igarashi K, Ichiyanagi K, Watanabe H, Kawasaki T, Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetesDiabet Med 2005 22(8):980-85. [Google Scholar]
[25]. Arsad SS, Esa NM, Hamzah H, Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) schott extractJ Cytol Histol S 2014 4(1):1-6. [Google Scholar]
[26]. Sharma P, Bodhankar SL, Thakurdesai PA, Protective effect of aqueous extract of Feronia elephantum correa leaves on thioacetamide induced liver necrosis in diabetic ratsAsian Pac J Trop Biomed 2012 2(9):691-95. [Google Scholar]
[27]. Yadav SK, Reddy BV, Sharma PL, Possible involvement of leptin in a Mas-receptor agonist, AVE-0991-induced improvement in dyslipidemia and cardiomyopathy in STZ-induced diabetic ratsJ Appl Pharm Sci 2013 3(11):70-75. [Google Scholar]
[28]. Aref AB, Ahmed OM, Ali LA, Semmler M, Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and Beta-cell function in the offspringJ Diabetes Res 2013 2013:01-10. [Google Scholar]
[29]. Geer EB, Islam J, Buettner C, Mechanisms of glucocorticoid-induced insulin resistance: Focus on adipose tissue function and lipid metabolismEndocrinol Metab Clin North Am 2014 43(1):75-102. [Google Scholar]
[30]. Burén J, Liu HX, Jensen J, Eriksson JW, Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytesEur J Endocrinol 2002 146(3):419-29. [Google Scholar]
[31]. Chien KL, Lee YT, Sung FC, Hsu HC, Su TC, Lin RS, Hyperinsulinemia and related atherosclerotic risk factors in the population at cardiovascular risk: A community-based studyClin Chem 1999 45(6):838-46. [Google Scholar]
[32]. Laakso M, Sarlund H, Salonen R, Suhonen M, Pyorala K, Salonen JT, Asymptomatic atherosclerosis and insulin resistanceArterioscler Thromb Vasc Biol 1991 11(4):1068-76. [Google Scholar]
[33]. Iida KT, Kawakami Y, Suzuki M, Shimano H, Toyoshima H, Sone H, Effect of thiazolidinediones and metformin on LDL oxidation and aortic endothelium relaxation in diabetic GK ratsAm J Physiol Endocrinol Metab 2003 284(6):E1125-30. [Google Scholar]
[34]. Shah SS, Chorawala MR, Shah GB, Anti-atherosclerotic potential of pioglitazone and N-acetylcysteineInt J Pharma Sci Drug Res 2012 3(2):434-42. [Google Scholar]
[35]. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H, Atherosclerosis: Process, indicators, risk factors and new hopesInt J Prev Med 2014 5(8):927-46. [Google Scholar]
[36]. Araki T, Emoto M, Teramura M, Yokoyama H, Mori K, Hatsuda S, Effect of adiponectin on carotid arterial stiffness in type 2 diabetic patients treated with pioglitazone and metforminMetabolism 2006 55(8):996-1001. [Google Scholar]
[37]. Eguchi K, Tomizawa H, Ishikawa J, Hoshide S, Numao T, Fukuda T, Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetesHypertens Res 2007 30(1):23-30. [Google Scholar]
[38]. Portillo-Sanchez P, Cusi K, Treatment of nonalcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitusClin Diabetes Endocrinol 2016 2(1):1-9. [Google Scholar]
[39]. Vikram A, Jena G, Ramarao P, Pioglitazone attenuates prostatic enlargement in diet-induced insulin-resistant rats by altering lipid distribution and hyperinsulinaemiaBr J Pharmacol 2010 161(8):1708-21. [Google Scholar]
[40]. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Role of AMP-activated protein kinase in mechanism of metformin actionJ Clin Invest 2001 108(8):1167-74. [Google Scholar]
[41]. Aghamohammadzadeh N, Niafar M, Dalir Abdolahinia E, Najafipour F, Mohamadzadeh Gharebaghi S, Adabi K, The effect of pioglitazone on weight, lipid profile and liver enzymes in type 2 diabetic patientsTher Adv Endocrinol Metab 2015 6(2):56-60. [Google Scholar]
[42]. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE, Metabolic effects of metformin in non-insulin-dependent diabetes mellitusN Engl J Med 1995 333(9):550-54. [Google Scholar]
[43]. Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trialJAMA 2008 299(13):1561-73. [Google Scholar]
[44]. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Long term pioglitazone treatment for patients with non alcoholic steatohepatitis and prediabetes or Type 2 diabetes mellitus: A randomized trialAnn Intern Med 2016 165(5):305-15. [Google Scholar]
[45]. Szapary PO, Bloedon LT, Samaha FF, Duffy D, Wolfe ML, Soffer D, Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndromeArterioscler Thromb Vasc Biol 2006 26(1):182-88. [Google Scholar]
[46]. Suman RK, Mohanty IR, Maheshwari U, Borde MK, Deshmukh YA, Metformin ameliorates diabetes with metabolic syndrome induced changes in experimental ratsInt J of Biomed & Adv Res 2016 7(2):55-65. [Google Scholar]
[47]. Hwang JL, Weiss RE, Steroid-induced diabetes: A clinical and molecular approach to understanding and treatmentDiabetes Metab Res Rev 2014 30(2):96-102. [Google Scholar]
[48]. Sayehmiri K, Asadollahi K, Yaghubi M, Abangah G, Nurmohamadi H, The effect of pioglitazone and metformin on non-alcoholic fatty liver: A double blind clinical trial studyJ Bas Res Med Sci 2014 1(1):50-55. [Google Scholar]
[49]. Naka KK, Papathanassiou K, Bechlioulis A, Pappas K, Kazakos N, Kanioglou C, Effects of pioglitazone and metformin on vascular endothelial function in patients with type 2 diabetes treated with sulfonylureasDiab Vasc Dis Res 2012 9(1):52-58. [Google Scholar]