Coronary Artery Disease (CAD) is one of the leading causes of non-communicable disease related deaths, both in developing and developed countries. CAD assumes significance among Indians, as India is reported to have the highest number of deaths in the world due to CAD [1]. By the year 2020, 2.6 million Indians are expected to die from CAD [2]. The 3-hydroxy-3-methylglutaryl co-enzyme A (HMGCoA) reductase inhibitors or statins have reduced the morbidity and mortality from CAD by effectively lowering Low Density Lipoprotein Cholesterol (LDL-C) [3], and offering cardioprotection through other pleiotropic mechanisms [4]. Statins decrease coronary events by 23% [5]. Atorvastatin is a safe and well tolerated lipid lowering agent and is said to be associated with fewer muscle and renal adverse effects compared to its congeners [6], Gastro-intestinal, hepatic, nervous system adverse effects (headache, dizziness, depression, peripheral neuropathy, cognitive impairment) elevations in HbA1C and fasting serum glucose, pancreatitis, anaphylaxis, angioneurotic oedema and bullous rashes are some of the adverse effects associated with atorvastatin use [6,7]. The sales of atorvastatin increased by 14.9% in 2015 [8], and is said to further increase with the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines identifying more patients eligible for statin therapy without clinically overt Atherosclerotic Cardiovascular Disease (ASCVD), based on the ASCVD risk scoring [9]. As more and more clinicians have started prescribing statins, the clinical problem of statin intolerance is becoming evident [10]. Overall, significant adverse effects of atorvastatin reported in large randomized clinical trials are at a frequency of less than 5% [11]. This may not be true in real life practice as clinical trials tend to underestimate the prevalence of ADRs [12], and the majority of the ADRs are under-reported due to lack of knowledge and time to report the same. It has been found that 30% of patients who need statins, stop taking them due to the ADRs caused by these drugs [13]. To improve patient compliance, it is essential to know the various ADRs of atorvastatin, their prevalence, risk factors for their development and their management. Hence, the aim of the present study was to report the AEs associated with atorvastatin use, their causality and severity in dyslipidemic South Indian Tamils, who differ in their genetic makeup from the rest of the Indian population [14,15].
Materials and Methods
Study Setting
The present cross-sectional study was carried out from October 2011 to April 2016, at a tertiary care teaching hospital- Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, located in Southern India. The study enrolled outpatients attending Cardiology and Medicine outpatient clinics of our hospital, who gave written consent to participate in the study. The study was approved by our Institute Scientific and Ethics Committee (JIP/IEC NO: EC/2011/4/6. dt 24.10.2011).
Dyslipidemic subjects of Tamil ethnicity (age between 30-65 years) with one or more additional risk factors (diabetes, hypertension, family history of premature CAD, low HDL cholesterol levels, smoking) for atherosclerotic cardiovascular disease were enrolled for the study. Dyslipidemia was defined as low density lipoprotein cholesterol of greater than 130 mg/dL, and if the patients were either diabetic or had CAD or both then, LDL-C greater than or equal to 100 mg/dL was also defined as dyslipidemia [16].
Those on any lipid lowering therapy within one month before study enrolment, those with contraindications to statin therapy (impaired hepatic or renal function, pregnant and lactating women), hypothyroid patients, those with LDL cholesterol >250 mg/dL or serum triglycerides >400 mg/dL and patients who were on drugs which modulate Cytochrome P 450 3A4/5 (CYP3A4/5) activity were excluded from the study.
Demographic characteristics, clinical history, concurrent medications, physical activity/lifestyle, dietary habits, anthropometric measures such as height, weight, Body Mass Index (BMI), Waist Hip Ratio (WHR) and biochemical parameters such as Fasting Blood Sugar (FBS) and fasting lipid profile (after overnight fast for at least 12 hours), Aspartate Transaminase (AST), Alanine Transaminase (ALT), blood urea nitrogen, serum creatinine and Creatine Kinase (CK) were recorded at baseline. Patients meeting eligibility criteria were started on atorvastatin treatment (dose ranging from 10-40 mg) by the treating clinicians and were encouraged to continue the treatment. Patients were asked to report after 45 days of atorvastatin treatment for follow up. They were enquired about new onset AEs linked to atorvastatin use {gastrointestinal disturbances headache, central nervous system disturbance, sleep disorders, myalgia, myopathy, rhabdomyolysis and hepatotoxicity} [17] and were also encouraged to report any other AEs which occurred during the study period. Treatment of coexisting hypertension, diabetes mellitus and coronary artery disease (drugs and their doses) was not allowed to be changed during the study period of 45 days, unless it was deemed urgent and essential by the treating physician during the study. Adherence to atorvastatin treatment was assessed by questioning and pill counting. Good adherence was defined as intake of medication for ≥36 days (i.e., 80% adherence), during 45 day study period [18]. All biochemical tests were repeated at the end of 45 days. Causality assessment for atorvastatin induced AEs was done using Naranjo adverse drug reaction probability scale criteria [19] and severity assessment was done using Hartwig scale [20]. AEs which were causally related to atorvastatin use were reported as ADRs. Muscle symptoms were classified as myalgia when there was no accompanying CK increase or CK increase of less than 4 times Upper Limit of Normal (ULN) [21].
Statistical Analysis
Continuous study variables were expressed as mean ± standard deviation when the data followed normal distribution and as median (interquartile range) when it was non-normally distributed. Frequencies were reported as percentages. Pearson’s Chi-square test/Fisher’s exact test was used to find the association of categorical variables with AEs. Wilcoxcon matched-pairs signed–rank test was used to compare statistical difference between pre and post treatment biochemical parameters such as Fasting Blood Glucose (FBS), AST, ALT and CK levels. A p-value of <0.05 was used as the level of significance throughout the study. All statistical tests were done using GraphPad InStat software version 3.6, La Jolla California, USA.
Results
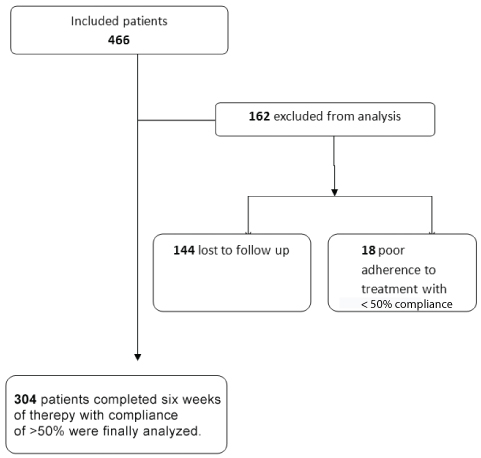
A total of 466 patients were recruited for the study, out of which five subjects (1.07%) discontinued treatment within first three weeks of treatment initiation. Out of these five patients, four had severe myalgia and one had discontinued it because of diarrhea. These subjects were excluded from the analysis and the cause of discontinuation of treatment was discerned only through telephonic interview. In the final analysis 304 patients were included who completed the study with 50% or greater adherence [Table/Fig-1]. The mean age of the study population was 49.87±9.04 years. Majority of the study participants were males (76.32%), overweight/obese (74.01%), and diabetics (63.82%). The mean BMI of our study participants was 25.87±4.24 kg/m2. The mean WHR was 0.91±0.05 for males, and 0.84± 0.04 for females. The other salient demographic characteristics of the study population are summarized in [Table/Fig-2].
Flow chart showing patient recruitment.
Demographic characteristics of the study patients (n=304).
| Patient Characteristics | N | Percentage (%) |
|---|
| Male:Female | 232/72 | 76.32/23.68 |
| Hypertensives | 155 | 50.98 |
| Diabetics | 194 | 63.82 |
| Overweight/Obese* | 225 | 74.01 |
| CAD patients | 30 | 9.86 |
| Current alcohol users | 79 | 25.98 |
| Inactive /sedentary lifestyle | 86 | 28.28 |
| Medication used by >10% of study patients |
| Metformin | 163 | 53.62 |
| Enalapril | 90 | 29.61 |
| Amlodipine | 68 | 22.37 |
| Glibenclamide | 48 | 15.79 |
| Glimiperide | 43 | 14.14 |
| Omeprazole | 43 | 14.14 |
| Aspirin | 41 | 13.49 |
| Insulin | 36 | 11.84 |
| Tablet B-Complex | 32 | 10.53 |
| Amitriptyline | 32 | 10.53 |
BMI ≥23kg/m2.
BMI = Body mass index; CAD = Coronary artery disease
Prevalence of Adverse Events
A total of 183 adverse events were noted in 145 (47.7%) patients. More than one AE was observed in 32 patients (22.07%). Overall AEs were significantly more common among females compared to males {60% (43/72) versus (vs) 44% (102/232), p=0.0194 chi-square test}. There was a statistically significant positive correlation between dose of atorvastatin and AEs (p=0.0208, Chi-square test). The commonest adverse events observed were myalgia (40.98%) followed by symptoms related to the nervous system and gastro-intestinal symptoms. [Table/Fig-3] shows the prevalence of AEs noted with atorvastatin use in our population and [Table/Fig-4] shows the distribution of AEs according to system. In our study, 72 out of 75 patients with myalgia had no CK elevation. Whole body ache was reported by majority of patients, followed by pain in the shoulder and arms. Females had a higher prevalence of myalgia compared to males (32% versus 22.5%) but the difference was not statistically significant (p=0.101). Myopathy and rhabdomyolysis were not observed in the present study.
Adverse events observed with atorvastatin use. (Number of reports=183).
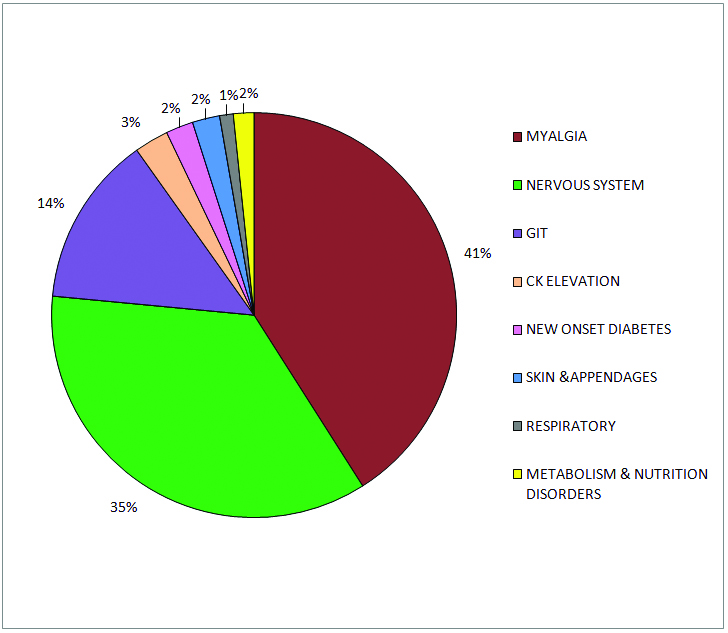
Distribution of adverse events according to system in the study population (n=304).
| System | ADRs |
|---|
| n | Percentage |
|---|
| Central nervous System |
| Headache | 17 | 5.6 |
| Giddiness | 17 | 5.6 |
| Peripheral neuropathy | 16 | 5.3 |
| Disorders of sleep | 12 | 3.9 |
| Sleep disturbanceExcessive sleep | 66 |
| Slurred speech | 1 | 0.3 |
| Memory impairment | 1 | 0.3 |
| Dizziness | 1 | 0.3 |
| Gastro-Intestinal System |
| Flatulence | 9 | 3 |
| Epigastric pain | 4 | 1.3 |
| Gastritis | 4 | 1.3 |
| Constipation | 2 | 0.6 |
| Dryness of mouth | 1 | 0.3 |
| Difficulty swallowing | 1 | 0.3 |
| Abdominal pain | 1 | 0.3 |
| Nausea | 1 | 0.3 |
| Vomiting | 1 | 0.3 |
| Diarrhea | 1 | 0.3 |
| Musculoskeletal System |
| Myalgia | 75 | 24.6 |
| Muscle pain | 53175 | 17.45.61.6 |
| Soreness/Tiredness |
| Non -specific /Joint pain |
| Nutrition and Metabolism |
| Increased appetite | 1 | 0.3 |
| Weight gain | 2 | 0.6 |
| Respiratory System |
| Cough | 1 | 0.3 |
| Rhinitis | 1 | 0.3 |
| Skin and Integumentary System |
| Itching | 4 | 1.3 |
| Others |
| New Onset Diabetes Mellitus (NOD) | 4 | 1.3 |
| Creatine kinase elevation | 5 | 1.6 |
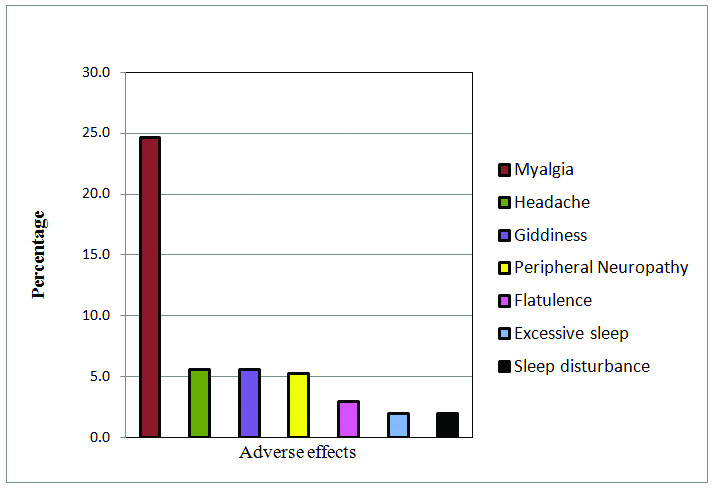
Apart from myalgia, the other notable AEs which were seen in >2% of the study population were headache, giddiness, peripheral neuropathy, flatulence, excessive sleep and sleep disturbance [Table/Fig-5]. New Onset Diabetes (NOD), was observed in a small percentage of our study population after 45 days of atorvastatin treatment, however there was no significant increase in FBS with atorvastatin treatment.
Adverse events occurring in at-least 2% of the study population.
Laboratory Abnormalities
CK elevation: Atorvastatin treatment in our population was associated with significant increase in CK levels, pre vs post-treatment levels were 108 (82) vs 120 (88) U/L (median {IQR}) -p < 0.001, CK levels were elevated between 3-4 times the ULN in five patients. Out of the five patients who had elevated CK levels, symptoms of myalgia were reported by three patients and two patients were asymptomatic. Severe myalgia with CK elevation leads to discontinuation of atorvastatin in one patient and dose reduction in another.
Elevated transaminases: No elevation in serum transaminases levels was found in our patient population. Pre-treatment vs post-treatment AST levels (median {IQR}) were 23 (14) vs 24 (13) U/L. A p-value =0.174. Similarly, there was no significant increase in serum ALT levels too. Pre vs post-treatment ALT levels were (median {IQR}) 23(15) vs 23(66) p=0.3269.
Atorvastatin Discontinuation and Dose Reduction
Among the patients who had ≥ 50% compliance to atorvastatin treatment (n=304), 145 patients (47.7%) developed AEs and of them 12 patients (4%) discontinued atorvastatin by 45 days of treatment. AE related atorvastatin discontinuation resulted from the following myalgia led to discontinuation in 3.45% (5/145), nervous system AEs in 2.76% (4/145), diarrhea in 0.69% (1/145) and multiple AEs in 1.38% (2/145) of the patients. Overall 9.5% (29/304) of patients required dose reduction due to AEs. Reasons for dose reduction among those who had AEs to atorvastatin were of myalgia in 4.83% (7/145), nervous system AEs 6.90% (10/145), 0.69% (1/145) each due to respiratory and G.I ADRs and two or more AEs were responsible for dose reduction in 6.90% (10/145) of the patients.
Causality and Severity of the Adverse Events
Causality of adverse events to atorvastatin treatment was assessed using Naranjo ADR probability scale which assessed whether the adverse effects were definitely, probably or possibly associated with the drug or it is doubtful. All of the observed adverse events were causally related to atorvastatin use (either probably or possibly associated with it) [Table/Fig-6] and hence termed ADRs. All the adverse events were mild, being of level 1 and 2 by the Hartwig severity assessment scale [Table/Fig-7].
Causality assessment of adverse events (Naranjo algorithm).
| ADR Score | n=183 | Percentage (%) |
|---|
| ≤0 – Doubtful | 0 | 0 |
| 1-4 Possible | 163 | 89.07 |
| 5-8 Probable | 20 | 10.93 |
| ≥9 = Definite | 0 | 0 |
Severity assessment of adverse events (Hartwig scale).
| Severity Level | n=145 | Percentage % |
|---|
| Level 1 | 104 | 71.72 |
| Level 2 | 41 | 28.27 |
| Level 3 | 0 | 0 |
| Level 4 | 0 | 0 |
| Level 5 | 0 | 0 |
| Level 6 | 0 | 0 |
| Level 7 | 0 | 0 |
Myalgia Causality and Severity Assessment
Causality assessment of myalgia was done using Naranjo adverse drug reaction probability scale criteria. The causality assessment revealed that 16% of the myalgia was probably due to atorvastatin use (scores 5-8) and 84% of myalgia was possibly due to atorvastatin treatment (scores 1-4) [Table/Fig-8]. The severity of myalgia, as assessed by Hartwig’s scale was level 1 in 84% (63/75) and level 2 in 16% (12/75) [Table/Fig-9].
Naranjo score for statin related myalgia.
| ADR Score | n=75 | Percentage (%) |
|---|
| ≤ 0 – Doubtful | 0 | 0 |
| 1-4 Possible | 63 | 84 |
| 5-8 Probable | 12 | 16 |
| ≥= Definite | 0 | 0 |
Severity assessment of myalgia (Hartwig scale).
| Severity Level | n=75 | Percentage (%) |
|---|
| Level 1 | 63 | 84 |
| Level 2 | 12 | 16 |
| Level 3 | 0 | 0 |
| Level 4 | 0 | 0 |
| Level 5 | 0 | 0 |
| Level 6 | 0 | 0 |
| Level 7 | 0 | 0 |
Discussion
In the present study, we were able to identify the proportion of AEs and also their causality to atorvastatin treatment in 304 dyslipidemic South Indian Tamils, which is the largest Indian study analysed for atorvastatin safety data. Randomized controlled trials with atorvastatin report good tolerability with a low incidence of adverse effects of ≤10% [11,22,23]. Pooled analysis of completed trials as well as clinical studies in various populations report the incidence of atorvastatin induced AEs to be between 18–29.4% [24-26], whereas, nearly half of patients in the present study developed ADRs. The higher proportion of AEs in the present study could be due to assessment of multi-system ADRs in addition to the differences in the race and ethnicity of the patients and the larger sample size.
Although there is no universal consensus on the definition of SRM, it is the most commonly reported AE from statin use. Randomized controlled trials have reported muscle related event rate of 1%–5% among statin users, similar to the control group [27]. These percentages tend to be on the lower side while observational studies report a higher incidence of muscle related events up to 33% among atorvastatin treated patients [28]. The proportion of myalgia in the present study is in concordance with the above literature. Two earlier Indian studies which reported AEs of atorvastatin therapy have documented occurrence of myalgia in 1%-6% of patients [29,30]. In one of these, myalgia occurred in patients taking 10 and 20 mg atorvastatin [29], and in another with atorvastatin 80 mg alone and not with 40 mg dose [30]. Myalgia was reported with all three doses (10, 20 and 40 mg) in the present study. Some of the factors contributing to increased risk of myalgia such as older age, small body frame, frailty, liver or kidney disease, hypothyroidism, alcoholism and excessive physical activity [10] could not explain the higher proportion of myalgia in our population. However, Asian race is a predisposing factor for statin related myalgia [31].
Nervous system AEs occurred at a higher frequency with atorvastatin use in the present study compared to previous Indian studies [29,30]. Gastrointestinal AEs, which have been reported as the predominant AE in earlier studies, were the third most common AE and occurred at a lesser frequency in the present study [24,25], AEs of respiratory tract, skin and appendages [32] were observed at lesser frequency with atorvastatin use in our population.
Food and Drug Administration (FDA) of United States in 2012 added a warning on labels that there may be an increase in fasting plasma glucose/ HbA1C with statins [33]. Kohli P et al., described New Onset Diabetes (NOD) as “at least two fasting plasma glucose ≥126 mg/dL after statin treatment initiation or at least one FPG ≥36 mg/ dL above pre-treatment value.” [34]. In the present study, NOD was documented in 1.3% of patients. This is lower compared to what has been reported earlier [35], most probably because of short duration follow-up in the present study. In the present study, even low to moderate dose atorvastatin treatment was associated with NOD, while in other studies the risk of NOD was found with higher dose of atorvastatin [36]. Weight gain with statin therapy was found to be an independent predictor of NOD [35]. In our study weight gain was observed in two patients, but it was not associated with NOD. Increased appetite with atorvastatin use, as seen in present study, has been reported earlier [37]. Elevated hepatic transaminases is said to occur in less than 1% of the patients with atorvastatin treatment [6]. In our study, none had an AST/ALT elevation greater than 3 times ULN.
Atorvastatin discontinuation because of AEs in our study was similar to few previous studies [6,25]. However, higher rates of atorvastatin discontinuation have been reported among patients above 65 years [6] and diabetic patients because of AEs [22]. The predominant AE responsible for atorvastatin discontinuation in the present study was myalgia, as reported in an earlier study [24].
Statins have been reported to cause more AEs in elderly patients[15] and females [38]. In the present study, females had significantly higher rate of AEs, but there was no association between age and AEs. Indian patients develop CAD a decade earlier compared to westerners and are started on statin treatment at much earlier age for CAD protection [39]. Development of AEs even among younger Indian patients, in a time span of 45 days as seen in the present study, is a cause for concern as it may interfere with their long term statin compliance.
Limitation
We could not assess the AEs of atorvastatin over longer duration of treatment; hence we did not observe long term adverse effects of atorvastatin such as hepatotoxicity in our study. Nearly one third of patients enrolled initially could not complete the study and hence were excluded from final analysis. Drug dechallenge and rechallenge studies were not possible due to time and monetary constraints.
Conclusion
Atorvastatin use among dyslipidemic South Indian Tamils was associated with increased frequency of adverse effects than previously reported among Indians. Atorvastatin-induced myalgia represents a major adverse effect in our population, which might threaten patient adherence to atorvastatin treatment and might increase their vulnerability to CAD related mortality and morbidity. Hence, there is an urgent need for mechanistic and molecular studies to find out the reason for these adverse drug effects in dyslipidemic South Indian Tamils which is unique from the rest of the world.
Funding: This study was supported by the financial aid from JIPMER intramural funds for the start- up research and was completed with the financial support by Department of Biotechnology, Government of India – Project San No-BT/PR5130/MED/12/553/2012.
Declaration
This study is a part of larger study assessing the influence of genetic variants on the lipid lowering efficacy of atorvastatin among dyslipidemic Tamilian population.(Indumathi C et al., Pharmacogenomics of Lipid Lowering Response of Atorvastatin among Dyslipidemic Tamilian Population [unpublished Ph.D thesis].)
BMI ≥23kg/m2.
BMI = Body mass index; CAD = Coronary artery disease