The use of stimulant drugs is increasing especially among teenagers who often experience significant psychological crisis [1,2], which may increase risky behaviours and drug abuse [3]. Many students abuse methylphenidate to improve their study performance [4,5] since they believe that it improves their memory, focus, attention and school activities [6]. Methylphenidateis also one of the most commonly prescribed drugs for attention deficit hyperactivity disorder [7,8], which is the most common behavioural disorder among children [9]. It is characterized by poor impulse control, hyperactivity and in attention, which impair school activities and social behaviour [10].
Methylphenidate as a psychostimulant drug increases the extracellular Dopamine (DA) and Norepinephrine (NE). It inhibits the reuptake of DA and NE by blocking the DA and NE Transporters (DAT, NET) [11]. The DAT is the main target of methylphenidate [12].
Evidence shows that deletion of DAT gene causes defects in skeletal integrity and structure [13]. Pharmacological blockade of NET also decreases NE reuptake in NET resulting in defective bone formation and increased bone resorption; thus, it possibly has an impact on bone mass [14].
Considering the confirmed effect of methylphenidateon calcium and phosphorus metabolism as well as bone homeostasis [13], it may play a role in tooth movement and bone remodeling as well. Numerous studies have indicated the role of methylphenidate in growth deficiency in patients under methylphenidate treatment [15-17].
Since most orthodontic patients are teenagers and children and considering the high consumption rate of methylphenidate by this age group, this animal study was conducted to assess the possible effect of methylphenidate consumption on orthodontic tooth movement in rats.
Materials and Methods
The study protocol was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences (No:IR.SSU.REC.1394.95), Yazd, Iran.
In this Experimental animal study, 42 male Wistar rats in the same age weighting 250 g-300 g were obtained from the animal room of the School of Medicine of Yazd University of Medical Sciences. The rats were housed in plastic cages for one week with a standard 12 hour light-dark cycle at 22±2ºC temperature and 55% moisture. The rats were fed soft food and standard diet containing 0.8%-1.2% calcium, 0.7%-0.9% phosphorus and vitamin D, and had access to sufficient amount of water. The study was conducted in accordance with the guidelines of the US National Institute of Health for the care and use of laboratory animals [18].
The rats were weighed at the onset of the study and were randomly divided into three equal groups (14 rats in each group). We used the formula below to convert the dose of methylphenidate in rat to an equivalent dose in human [19]:

The rat and human CFs are 6 and 37, respectively. Based on these data [19], doses of 3, 6, 9 and 12 mg/kg in rats are similar to 0.47, 0.97, 1.45 and 1.94 mg/kg, respectively in humans.
The Group 1 received 5 mL/kg water by gavage. The rats in the experimental Group 2 received a constant dose of 3 mg/kg/day of methylphenidate for 14 day and the rats in the experimental Group 3 received increasing doses of methylphenidate hydrochloride by gavage, starting with 3 mg/kg/day on days one to four, followed by 6 mg/kg/day on days five to eight, 9 mg/kg/day on days 9 to 12 and 12 mg/kg/day on days 13 and 14. The drug was administered along with 5 mL/kg of water by gavage.
Orthodontic Appliance Placement
A 44 mg/kg ketamine hydrochloride (Gedeon Richter Ltd, Budapest, Hungary) and 2 mg/kg xylazine (Rompoun, Bayer, Leverkusen, Germany) were injected with intraperitoneal method to anaesthetized the rats [3]. To apply orthodontic force, a 5 mm NiTi closed coil spring (NiTi, 3M Unitek, Monrovia, CA, USA) and ligature wire (Dentaurum group, Ispringen, Germany) were used.
Orthodontic springs were placed in all experimental groups. The spring was placed between the maxillary right first molar and the maxillary right central incisor. Activation of the spring by 2 mm-5 mm delivered about 30 g of force to the first molar and central incisor. To obtain greater retention on the cone-shaped incisor, we created cervical grooves on the labial and distal surfaces of the right central incisor using a diamond bur [Table/Fig-1].
Cervical grooves on the labia and distal surfaces of the right central incisor.

After etching and rinsing, small amount of light-cure composite resin was applied on the ligature wire and it was tied around the tooth at the location of the cervical grooves [Table/Fig-2].
Application of nickel-titanium closed-coil spring.

Measurement of Orthodontic Tooth Movement
We measured the movement of first molar 14 days after the placement of orthodontic appliance because bone remodeling requires 10-14 days to take place [20,21].
At the end of the experimental period on day 14, all animals were weighted and their general condition was evaluated. The rats were anesthetized with overdose of ether for 15 minute in a plastic container then animals decapitated with a scalpel. The maxilla was resected. The amount of tooth movement was measured between the first and second molars using an interproximal filler gauge, which was calibrated in increments of 0.05 mm [Table/Fig-3,4]. All measurements were repeated twice. After measurement of tooth movement, premaxillae of the rats were placed in 10% formalin. The samples were fixed for 10 days and were then decalcified in 5% formic acid for six days. The decalcified samples were embedded in paraffin. To have a control for bone resorption due to orthodontic spring placement, the bone resorption around the corresponding teeth in the contralateral side of the arch was evaluated in all experimental groups.
Measurement of tooth movement using an interproximal filler gauge.

Interproximal filler gauge, which was calibrated in increments of 0.05 mm.

Histological Analysis
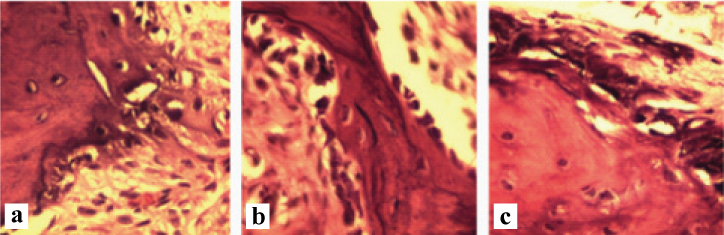
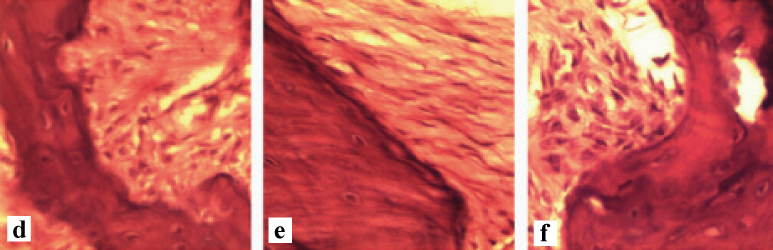
Paraffin blocks were sectioned in a parasagittal plane at the site of the mesiobuccal root of the first molar with 4 µm-5 µm thickness. The samples were then stained with haematoxylin and eosin and histological features evaluated under a light microscope (Olympus Bx-41). The number of osteoclasts and resorption lacunae and maximum depth of each resorption lacuna were calculated to evaluate the amount of root resorption [Table/Fig-5,6a-c,d-f].
A histopathological section of the alveolar bone of rat upper first molar tooth (×40) showing osteoclasts and Howship’s lacunae (haematoxylin and eosin).

A histopathological section of the alveolar bone with exerted force (×40). a) Group 1; b) Group 2; c) Group 3.
A histopathological section of the alveolar bone without exerted force (×40) d) Group 1; e) Group 2; f) Group 3.
Statistical Analysis
Differences between the experimental groups were analysed using Kruskal-Wallis and Mann–Whitney U Tests. In this study, the mean value was reported for orthodontic tooth movement, number of osteoclasts, number of resorption lacunae and maximum depth of resorption lacunae in the experimental groups.
The p<0.05 was assumed statistically significant. All statistical analyses were performed using SPSS version 16.0 software.
Results
Orthodontic Tooth Movement
At the end of the study period, seven rats were excluded because of detachment of orthodontic appliance or weight loss (less than 250 mg). Two rats in Group 1 and 2, three rats in Group 3 were excluded.
All experimental groups with orthodontic spring showed evidence of tooth movement [Table/Fig-7].
Descriptive statistics of orthodontic tooth movement after 14 days.
| Groups | N | Mean (mm) | SD | SE | minimum (mm) | maximum (mm) |
|---|
| 1 | 12 | 0.2042 | 0.1117 | 0.3225 | 0.05 | 0.4 |
| 2 | 12 | 0.1750 | 0.1158 | 0.3343 | 0.05 | 0.35 |
| 3 | 11 | 0.1091 | 0.0436 | 0.0131 | 0.05 | 0.2 |
SD: Standard deviation; SE: Standard error
Group 1: Control group. Group 2: Received constant doses of methylphenidate; Group 3: Received increasing doses of methylphenidate.
The Group 1 showed the greatest tooth movement (mean value of 0.20 mm). The experimental Group 3 receiving increasing doses of methylphenidate showed the least movement (mean value of 0.10 mm). Kruskal–Wallis showed that there were no significant differences in the amount of orthodontic tooth movement between the experimental groups (p=0.072) [Table/Fig-8].
Kruskal wallis test significance for Group variables.
| Grouping Variable | p-value |
|---|
| Amount of Movement | 0.072 |
| Number of Osteoclast | 0.4 |
| Number of Lacuna | 0.16 |
| Depth of lacuna | 0.04 |
p-value<.05
Histological Findings
Osteoclasts were seen lining the mesial surface of the right first molar in all groups, but the difference in this regard was not statistically significant among the experimental groups (p=0.4) [Table/Fig-9]. An increase in the mean number of lacunae was found in the Group 1 compared to the methylphenidate groups [Table/Fig-10]; however, it was not statistically significant (p=0.16) (p>0.05). Kruskal–Wallis showed that there were significant differences among the three groups regarding the depth of resorption lacuna (p=0.04). Mann– Whitney Tests showed significant differences in the depth of resorption lacuna between Group 2 and 3.(p=0.037) [Table/Fig-11]. The lacunae in the experimental Group 3 taking increasing doses of methylphenidate were shallower than those in the other groups [Table/Fig-12].
Descriptive statistics of number of osteoclasts after 14 days.
| Groups | N | mean | SD | SE | Minimum | Maximum |
|---|
| 1 | 12 | 2.2500 | 0.8660 | 0.2500 | + 1 | +4 |
| 2 | 12 | 2.3333 | 0.9847 | 0.2842 | + 1 | +4 |
| 3 | 11 | 2.2727 | 0.7862 | 0.2370 | + 1 | +3 |
SD: Standard Deviation; SE: Standard Error
Group 1: Control group. Group 2: Received constant doses of methylphenidate; Group 3: Received increasing doses of methylphenidate.
+1: 1 to 3 number of osteoclast / hpf; +3: 5 to 8 number of osteoclast /hpf; +4: more than 8 number of osteoclast / hpf
Descriptive statistics of number of lacunae after 14 days.
| Groups | N | mean | SD | SE | Minimum | Maximum |
|---|
| 1 | 12 | 1.4167 | 0.5149 | 0.1486 | + 1 | +3 |
| 2 | 12 | 1.0833 | 0.9003 | 0.2599 | 0 | +3 |
| 3 | 11 | 1.0000 | 0.6324 | 0.1906 | 0 | +2 |
SD: Standard deviation; SE: Standard error
Group 1: Control group. Group 2: Received constant doses of methylphenidate; Group 3: Received increasing doses of methylphenidate.0: there was no lacunae; +1: 1 to 3 number of lacunae/ hpf; +2: 3 to 5 number of lacunae/ hpf; +3: 5 to 8 number of lacunae/hpf
Mann Whitney U test significance for intergroup comparison.
| Grouping Variable | P-value |
|---|
| Group 1,2 | 0.16 |
| Group 1,3 | 0.41 |
| Group 2,3 | 0.037 |
Group 1: Control group. Group 2: Received constant doses of methylphenidate; Group 3: Received increasing doses of methylphenidate. p-value<.05
Descriptive statistics of depth of lacunae after 14 days.
| Groups | N | Mean (mm) | SD | SE | Minimum (mm) | Maximum (mm) |
|---|
| 1 | 12 | 1.1667 | 0.5773 | 0.1666 | 0 | +2 |
| 2 | 12 | 0.7500 | 0.6215 | 0.1794 | 0 | +2 |
| 3 | 11 | 0.6364 | 0.8090 | 0.2439 | 0 | +2 |
SD: Standard deviation; SE: Standard error
Group 1: Control group. Group 2: Received constant doses of methylphenidate; Group 3: Received increasing doses of methylphenidate. 0: 0 mm depth of lacunae/ hpf; +2: 3 mm to 5 mm depth of lacunae/hpf
Histological evaluation of the contralateral arch (without force application) showed a reduction in the number of osteoclasts and lacunae as well as lacuna depth.
Discussion
Orthodontic tooth movement is a dynamic and inflammatory process involving alveolar bone remodeling and periodontal tissue rearrangement. Some drugs and systemic conditions affect the remodeling process of bone [22]. Many researchers evaluated the effect of drugs and synthetic materials on bone metabolism and orthodontic tooth movement. But to the best of authors’ knowledge, no previous study evaluated the effect of methylphenidate on bone remodeling and orthodontic tooth movement. We evaluated the effect of constant and increasing doses of methylphenidate on orthodontic tooth movement in rats to assess the effect of both therapeutic and higher doses of methylphenidate used by patients with risky behaviours such as drug abuse. The therapeutic dose of methylphenidate is 0.25-1 mg/day [23]. We performed the procedure suggested by the US food and drug administration for exact conversion of the human dose to a similar dose for use in rats. This method is based on the body surface area rather than the body weight only [19] because the latter method depends on several factors in mammalian biology such as basal metabolism, renal function and blood volume.
We found that methylphenidate (as inducer of extracellular DA and NE) had no significant effect on orthodontic tooth movement or bone resorption in rats.
However, Mann Whitney U tests revealed significant differences in the depth of resorption lacuna between Group 2 and 3 (p= 0.037), but this finding is not clinically important, because of no significant change in osteoclasts and lacunae.
However, there is no study on the effects of methylphenidate on orthodontic tooth movement to compare our results with. Several studies assessed the effect of methylphenidate on bone mass and bone mineral density [16,24]. Lahat E et al., demonstrated that no statistically significant difference existed in bone mineral density and turnover in children who received methylphenidate compared to the control group, which was although in agreement with our findings [14]. But, Komatsu DE et al., showed that long-term administration of methylphenidate in young rats resulted in less mineralized and weaker appendicular bones [16]. Several studies indicated the effect of DAT and NET on bone metabolism [13,14,25]. Bliziotes M et al., demonstrated that mice with homozygous deletion of DAT had reduced bone mass and strength as a result of decrease in cancellous bone volume [13]; the calcium content of the femur decreased in rats [26]. Deletion of DAT may alter mineral metabolism by an imbalance in regulation of absorption and secretion of calcium and phosphate. DA modulates phosphate secretion by the kidneys [26]. Although, DA inhibits phosphate transportation by regulating the Na1/Pi co-transport in proximal tubules of the kidneys [27,28], the levels of serum and urine calcium and phosphorus and parathormone were not significantly different between DAT-/- phenotype and control groups in a previous study [26].
Osteoclast recruitment is one factor indicative of bone resorption and tooth movement. The osteoclast resorption rate is regulated by metabolic factors, particularly parathormone. Because of no change in the level of parathormone [26], no significant change in bone resorption or orthodontic tooth movement is expected.
Some studies showed a decrease in NE reuptake resulting in defective bone formation and increased bone resorption [14]. However, another study assessed the effect of NET selective blockers on bone and found no significant effect on bone mineral density [29]. Thus, it may be concluded that although some studies indicate a temporary decrease in the rate of growth [30] no significant difference occurs in bone turnover or weight [24,30]. Changes in mineral density and bone turnover seem to have an insignificant effect on the clinical result and bone resorption. In the current study, histological analysis did not reveal any significant difference in the number osteoclasts or lacuna.
But in the experimental Group 3 (no force application), number of osteoclasts and lacunae and depth of lacuna decreased, which may indicate a dopaminergic effect on the count of osteoclasts and lacunae.
Limitation
This study was conducted on rats; therefore, the results may not be completely generalizable to humans. Further studies should evaluate the clinical and cytological changes in humans. Also, DA and NE may affect the activity of bone cells; therefore, future studies are required to assess minor changes in the activity of osteoclasts.
Conclusion
Methylphenidate likely did not affect orthodontic tooth movement or bone resorption; although it had a small insignificant effect on the number of osteoclasts and lacunae. Further studies are required to assess the effect of methylphenidate on orthodontic tooth movement and bone resorption in humans.