Oral Candidal Carriage in Subjects with Pure Vegetarian and Mixed Dietary Habits
Shankargouda Patil1, Roopa S Rao2, A Thirumal Raj3, D.S. Sanketh4, Sachin Sarode5, Gargi Sarode6
1 Associate Professor, Department of Maxillofacial Surgery and Diagnostic Sciences, Division of Oral Pathology, College of Dentistry, Jazan University, Jazan, Saudi Arabia.
2 Professor and Head, Department of Oral Pathology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India.
3 Senior Lecturer, Department of Oral Pathology and Microbiology, Sri Venkateswara Dental College and Hospital, Chennai, Tamil Nadu, India.
4 Oral Pathologist and Founder, HackDentistry.
5 Professor, Department of Oral Pathology and Microbiology, Dr. D. Y. Patil Dental College and Hospital, Dr. D.Y. Patil Vidyapeeth, Pimpri, Pune, India.
6 Reader, Department of Oral Pathology and Microbiology, Dr. D. Y. Patil Dental College and Hospital, Dr. D.Y. Patil Vidyapeeth, Pimpri, Pune, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. A Thirumal Raj, Postgraduate Student, Department of Oral Pathology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru-560054, Karnataka, India.
E-mail: thirumalraj666@gmail.com
Introduction
Candida albicans being a part of the normal oral microbial flora is one of the most commonly isolated species from the oral cavity. Recent studies have shown a steady rise in the number of non C. albicans species, which are relatively resistant to common antifungal agents and are being recognized as potential pathogens. It is vital to ascertain the predisposing factors leading to such a shift in the oral candidal flora.
Aim
To estimate the prevalence of candidal species among vegetarians and non-vegetarians.
Materials and Methods
Clinical data including age, gender, and diet preference of 238 participants were noted. Participants with a history of systemic disorders, oral prosthesis, salivary gland disorders and habits such as smoking, alcoholism, and tobacco usage were excluded from the study. The participants were asked to gargle a 10 ml solution of phosphate buffered saline for one minute before depositing the same in a sterile container. The samples were cultured using Hicrome agar media. Data analysis was carried out using Statistical Package for Social Sciences (SPSS software) version 10.5 and differences between individual groups were tested by Chi-square test.
Results
Among 238 samples, 127 (53.3%) samples were positive for Candida. The candidal prevalence in vegetarians (68.5%) was higher than non-vegetarians (40.7%). C. albicans was the most common species to be isolated in both vegetarians (35.1%) and non-vegetarians (39.2%). Candida glabrata and Candida tropicalis showed a higher prevalence in vegetarians (30.5% and 10.1%, respectively) in comparison to non-vegetarians (8.4% and 2.3%, respectively). Candida krusei was isolated only from vegetarians (4.6%).
Conclusion
Results indicate that diet plays a major role in oral candidal prevalence and species specificity which in turn may predispose the vegetarians toward these pathogenic organisms.
Agar, Candida, Diet, Oral health
Introduction
Candidal infections range from superficial lesion in an apparently healthy individual to life-threatening infection in an immune compromised patient. Since Candida is considered to be part of the normal oral microbial flora, it is vital to determine the factors predisposing an individual in developing candidal infections [1,2]. These factors may range from local alteration in the oral microbial flora due to smoking, alcoholism, compromised periodontal health to systemic conditions and drugs causing immune suppression. Previous studies have focussed on identifying the causal relationship between systemic disorders and associated habits with oral health [3]. Studies have shown a substantial relationship between dietary intakes to changes in the oral microenvironment. These changes range from an increased microbial retention to decreased buffering capacity of saliva [4,5].
A systematic review by Green R et al., identified major dietary patterns in India. Vegetarian diet consisting of vegetables, fruits, cereals and pulses was the most predominant dietary pattern. It was followed by a mixed dietary pattern consisting of high sugar, high fat and meat in addition to vegetables, fruits, cereals and pulses [6]. Our hypothesis was that variations in the dietary intake might have a substantial effect on the oral flora, which in turn may predispose an individual to retain the pathogenic non Candida albicans species. Therefore, current study investigates the colonization pattern of Candida species among vegetarians and non-vegetarians (mixed dietary habits).
Materials and Methods
The present study was a cross-sectional observational study conducted in the Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences from March 2015 to April 2015. Informed consent was obtained from all the participants and the scientific and ethics committee of the institute has approved this study. Vegetarians (n=108) and non-vegetarians (n=130) were selected from pool of 245 adults. Non-vegetarian inclusion criteria consisted of subjects consuming meat at least once in a week. All the participants belonged to the age range of 18-30 years. There were no statistically significant differences between the ages of the two groups. Participants with systemic disorders, visible oral lesions, dental caries, periodontal diseases, salivary gland disorders, oral prosthesis, restorations and with history of smoking, alcoholism, and use of tobacco products were excluded from the study as these areas could aid in the adherence of Candida species and may result in false positive result. Out of the 245 participants seven were excluded due to history of smoking and alcoholism.
Oral rinse was sampled as described by Leung WK et al., with slight modifications [7]. The participants were given 10 ml of phosphate buffered saline with a pH 7.2 in a sterile universal container. All the participants were asked to gargle the solution for 1 min and to deposit the same into the sterile container. The sample was then transferred onto a disposable centrifuge tube. All the samples were processed within 30 min of sampling.
Culture: The samples were centrifuged at 2000 rpm for 10 min. The resulting pellet in the bottom of the tube was isolated. Disposable inoculation loops were used to transfer the pellet onto the Hicrome agar plates. The culture plates were incubated for 24 hour at 37°C. All cultures were then examined and the total candidal prevalence and species specificity was assessed. Using HiCrome Candida Differential Agar M1297A.
Identification of isolates: The candidal species were identified based on the colour code provided by the manufacturer. Colonies of C. albicans were light green, C. glabrata were cream to white, C. krusei were fuzzy purple and C. tropicalis were blue to purple. The participant’s demographic and microbiological data were analysed, and the differences between individual groups were tested by Chi-square test. the p-value <0.05 was considered statistically significant. Data analysis was carried out using SPSS package (Version 10.5).
Results
The demographic data of the subjects are shown in [Table/Fig-1]. Almost, 53.3% of the subjects were positive for Candida. The overall candidal prevalence was higher in vegetarians (68.5%) than non-vegetarians (40.7%). The species isolated were Candida albicans, Candida tropicalis, Candida glabrata, and Candida krusei. The candidal species specificity among the vegetarians and non-vegetarians is shown in [Table/Fig-2]. The higher prevalence of non C. albicans species was noticed in vegetarians in comparison to non-vegetarians (Chi-square test, p<0.05), but the C. albicans species was found to have greater predilection towards non vegetarians than vegetarians. The light green colonies of C. albicans isolated from a non-vegetarian participant is depicted in [Table/Fig-3]. Such pure isolates of C. albicans were found to a greater extent among non vegetarians. Mixed colonies of C. krusei, C. glabrata, and C. albicans isolated from a vegetarian participant is depicted in [Table/Fig-4]. Mixed colonies of C. tropicalis and C. glabrata isolated from a vegetarian participant is depicted in [Table/Fig-5]. Among such mixed colonies, the dominant species in most cases of vegetarians were the non Candida albicans species.
Demographic data of the subjects.
| Diet | Demographics | Candidal positivity (%) |
|---|
| Age in years (Mean) | Gender(%) |
|---|
| Male | Female |
|---|
| Vegetarian (108) | 22.3 | 17 (15.8) | 91 (84.2) | 74 (68.5) |
| Non vegetarian (130) | 21.4 | 37 (28.5) | 93 (71.5) | 53 (40.7) |
| Total (238) | 21.85 | 54 (22.6) | 184 (77.3) | 127 (53.3) |
Candidal species specificity among the vegetarians and non-vegetarians.
| Diet | Candidal species |
|---|
| Candida albicans (%) | Non Candida albicans species (%) |
|---|
| C. albicans | C. tropicalis | C. glabrata | C. krusei |
|---|
| Vegetarian (108) | 38 (35.1) | 11 (10.1) | 33 (30.5) | 5 (4.6) |
| Non vegetarian (130) | 51 (39.2) | 3 (2.3) | 11 (8.4) | 0 |
| Total (238) | 89 (37.3) | 14 (5.8) | 44 (18.4) | 5 (2.1) |
C. albicans: Candida albicans, C. tropicalis: Candida tropicalis, C. glabrata: Candida glabrata, C. krusei: Candida krusei. Several vegetarian and non vegetarian patients showed mixed Candidal colonies (meaning an individual patient had two to three different Candidal species). Thus even if the patient count is one, the total species count may be more (depending on how many different species was present in the patient).
Light green Candida albicans colonies isolated from non vegetarian participant.
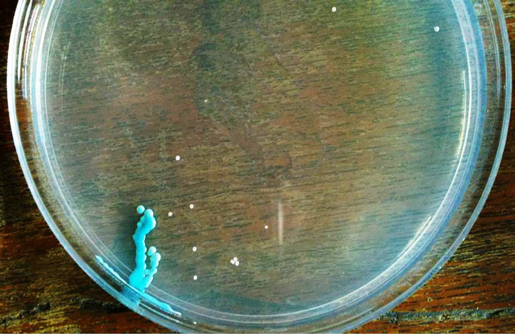
Mixed colonies of purple fuzzy Candida krusei and cream to white Candida glabrata isolated from vegetarian participant
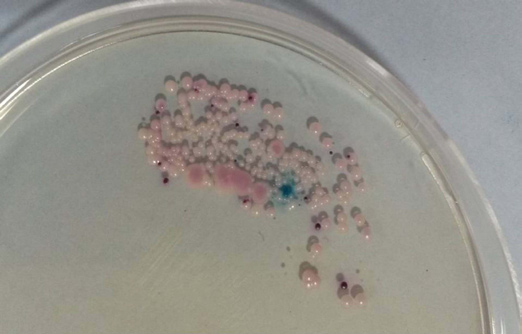
Mixed colonies of blue to purple Candida tropicalis and cream to white Candida glabrata isolated from vegetarian participant.

Discussion
In the present study, prevalence and species specificity of oral Candida in a group of vegetarians and non-vegetarians was investigated using a cross-sectional study design. The candidal colonization pattern of the subjects belonging to both the groups was assessed based on the colour coding provided by the manufacturer (HiCrome Candida Differential Agar M1297A). The total candidal prevalence and the predominant candidal species isolated were compared between the two groups. This study showed that a vegetarian diet predisposed the subject to a higher candidal prevalence and a non C. albicans species colonization. Various factors could have played a role in an increased sustenance of Candida in vegetarians including a diminished salivary flow, low pH, and a reduced buffering capacity. Linkosalo E et al., concluded that a change from mixed to a lacto-vegetarian diet decreased the flow rate and buffering capacity of saliva [5]. This is in contrast to the study by Johansson G et al., [4]. They noticed that a change from mixed to a lacto-vegetarian diet caused a substantial increase in the whole salivary flow rate and buffering capacity, but there was no significant rise in the number of lactobacilli or mutans streptococci.
Keten D et al., found a significant increase in the oral Candidal carriage in Maras powder users (54%) in comparison to non users (22%) [8]. A similarly increased rate of candidal colonisation was noted in smokers by Darwazeh AM et al., and in smokeless tobacco chewers by Javed F et al., [9,10]. Nicotine, the major incredient in tobacco is suggested to cause structural and functional alteration to oral keratinocytes, increasing their susceptibility to candidal species [11,12]. Further, smoking causes hyperkeratinisation of the epithelium, which in turn may lead to increased retention of Candidal organisms [13]. Similarly, the composition of the vegetarian/non-vegetarians food and/or the differences in the masticatory pattern (due to physical properties of the food like its texture, consistency etc.,) between the two diets may play a role in conditioning the epithelium towards predisposing/resisting the candidal species. Although our exclusion criteria has eliminated other possible causes of candidal retention like oral prosthesis, systemic disorders and associated habits, the individual’s genetic and epigenetic predispositions cannot be ignored.
Limitation
It represents a single point observation (cross-sectional) study. Long term follow up studies with periodic alteration in diet may aid in signifying the role of diet as an independent factor for the overall candidal prevalence and species specificity.
Conclusion
We postulate that diet-induced changes of the intraoral environment might have played a major role in the shift in the candidal species. This study shows an increased prevalence of non C. albicans species, which are considered to have a relatively higher drug resistance in comparison to C. albicans species. The question as to whether the reason for the increased prevalence and the shift in the candidal species lies solely on dietary differences or that additional factors could have played a role needs further appraisal.
C. albicans: Candida albicans, C. tropicalis: Candida tropicalis, C. glabrata: Candida glabrata, C. krusei: Candida krusei. Several vegetarian and non vegetarian patients showed mixed Candidal colonies (meaning an individual patient had two to three different Candidal species). Thus even if the patient count is one, the total species count may be more (depending on how many different species was present in the patient).
[1]. Scully C, El-Kabir M, Samaranayake LP, Candida and oral candidosis: A reviewCrit Rev Oral Biol Med 1994 5(2):125-57. [Google Scholar]
[2]. Ilze Messeir I, Pedro MD, Charlene WJ, Strengths and limitations of different chromogenic media for the identification of Candida speciesJ Microbiol Res 2012 2:133-40. [Google Scholar]
[3]. Birman EG, Kignel S, Da Silveira FR, Paula CR, Candida albicans: Frequency and characterization in oral cancer (Stage I) from smokers and drinkersRev Iberoam Micol 1997 14:101-03. [Google Scholar]
[4]. Johansson G, Birkhed D, Effect of a long-term change from a mixed to a lactovegetarian diet on human salivaArch Oral Biol 1994 39:283-88. [Google Scholar]
[5]. Linkosalo E, Ohtonen S, Markkanen H, Karinpää A, Kumpusalo E, Caries, periodontal status and some salivary factors in lactovegetariansScand J Dent Res 1985 93:304-08. [Google Scholar]
[6]. Green R, Milner J, Joy EJ, Agrawal S, Dangour AD, Dietary patterns in India: a systematic reviewBr J Nutr 2016 116(1):142-48. [Google Scholar]
[7]. Leung WK, Dassanayake RS, Yau JYY, Jin LJ, Yam WC, Samaranayake LP, Oral colonisation, phenotypic and genotypicprofiles of Candida species in irradiated, dentate, xerostomic nasopharyngeal carcinoma survivorsJ Clin Microbiol 2000 38:2219-26. [Google Scholar]
[8]. Keten D, Keten HS, Goktas MT, Ucer H, Ersoy O, Celik M, Oral Candida carriage and prevalence of Candida species among Maras powder users and non-usersJ Oral Pathol Med 2015 44:502-06. [Google Scholar]
[9]. Darwazeh AM, Al-Dwairi ZN, Al-Zwairi AA, The relationship between tobacco smoking and oral colonization with Candida speciesJ Contemp Dent Pract 2010 11:17-24. [Google Scholar]
[10]. Javed F, Tenenbaum H, Nogueira-Filho G, Oral Candida carriage and species prevalence amongst habitual gutka-chewers and non-chewersInt Wound J 2014 11(1):79-84. [Google Scholar]
[11]. Arredondo J, Nguyen VT, Chernyavsky AI, A receptor mediated mechanism of nicotine toxicity in oral keratinocytesLab Invest 2001 81:1653-68. [Google Scholar]
[12]. Soysa NS, Ellepola ANB, The impact of cigarette/tobacco smoking on oral candidosis: an overviewOral Dis 2005 11:268-73. [Google Scholar]
[13]. Williams DW, Walker R, Lewis MA, Allison RT, Potts AJ, Adherence of Candida albicans to oral epithelial cells differentiated by Papanicolaou stainingJ ClinPathol 1999 52:529-31. [Google Scholar]