Periodontitis is an inmmunoimflammatory disease that is characterized by the destruction of the attachment apparatus of the periodontium [1]. The mainstay aim of periodontal treatment is the regeneration of the lost attachment apparatus of the teeth. Variety of treatment modalities are available for periodontal regenerative therapy including bone grafts, bone substitutes, guided tissue regeneration, growth factors, application of tissue engineering or the combination of two or more of the above listed approaches [2]. Alloplasts, may be an effective alternative to allograft and xenografts as there is no risk of disease transmission and the supply is unlimited [3]. The bioactive glass enhances osteogenesis due to its properties of adsorption and concentrations of proteins that are utilized by osteoblast in order to form a mineralized extracellular matrix [4]. The advantage of the putty form of bioactive glass is that it contains glycerine and polyethylene glycol which makes the glass particle coherent and thus enhancing handling characteristics and minimal migration of graft particles from the defect site [5]. Histological evaluation of material has shown that the particulate tends to retard the down growth of epithelial tissue [6-9].
Growth factors play a pivotal role in periodontal regeneration. PRF is believed to release polypeptide growth factors, such as transforming growth factors-ß, platelet derived growth factors, vascular endothelial growth factors and matrix glycoproteins (such as thrombospondin -1) into the surgical wound in a sustained fashion for at least seven days as shown in vitro [10]. Thus, given the unique graft with osteoconductive, osteoinductive and osteostimulative propertiesand properties of autologous PRF, application of combination approach was attempted for the assessment of their additional benefits to the healing mechanisms and periodontal regeneration in intrabony defects.
The objectives of the study was to examine the efficacy of regenerative potential of graft material and to examine the ability of PRF to augment the regenerative effect of bioactive glass and these objectives were evaluated using probing depth reduction, clinical attachment gain and bone fill osseous defect.
Materials and Methods
This randomized controlled trial (NCT 02982681) was carried out in the Department of Periodontics and Oral Implantology, Santosh Dental College and Hospital, Santosh University, Ghaziabad, Uttar Pradesh, India. The institutional ethical committee approved the study.
Neither the patients nor the investigator was aware of the group assignment, thereby assuring double blindness.
Population Screening
A total of 20 patients were assessed for the eligibility of which 10 patients were selected based on the inclusion and exclusion criteria. A total of 20 bone defects (10 pairs) were selected using convenience sampling. The selected sites in each individual were randomly divided into control site and test site according to split mouth design technique.
Selection Criteria
Systematically healthy subjects, aged between 20 to 50 years (seven males and three females) suffering from moderate to severe localized chronic periodontitis, having radiographic evidence of one or more vertical defects (two or three walled) and probing pocket depth of 5 mm or more at the experimental site were enrolled [3]. Patients with systemic diseases, on anticoagulants, those with habit of smoking and alcohol, with known history of allergy to graft material and who have undergone periodontal surgical treatment for chronic periodontitis within twelve months for the same defects were excluded from the study. Pregnant and lactating females as well as patients on antibiotic therapy were also excluded from the study. The patients were explained about the procedure and a written informed consent was obtained.
Presurgical Therapy
Patients underwent phase I therapy which included oral hygiene instructions, scaling and root planing under local anaesthesia, and occlusal correction if trauma existed. Adjunctive chemical plaque control, in the form of chlorhexidine mouth rinse 0.12% twice daily, was advised. The selected defects were evaluated after two weeks, and persistent pockets > or = 5 mm, clinical radiographic evidence of angular osseous defects were scheduled for periodontal flap surgery.
Clinical Parameters
The sites to be grafted were assessed on the basis of evaluation of the selected clinical parameters which included, Gingival index (GI) (Loe and Sillness) [11], Plaque index (PI) of Silness and Loe [12] Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) were recorded by a single investigator, at baseline on the day of surgery, 3, 6 and 9 months intervals.
PPD and CAL were recorded using UNC-15 probe and customized acrylic occlusal stents grooved in the area of defect to provide reproducible insertion axis from:
Fixed Reference Point (FRP) to the Base of Pocket (BP)
Fixed Reference Point (FRP) to the Cemento-Enamel Junction (CEJ)
Fixed Reference Point (FRP) to the Gingival Margin (GM)
Radiographic measurements: Standardized intraoral periapical radiographs of the defects were taken using a paralleling technique [13].
Amount of defect fill: Defects were measured from the fixed reference point (distance between the CEJ to the radiographic base of the bone defect) with the help of 1.1 mm grid and the following radiographic features were recorded on the day of surgery, 3, 6 and 9 months intervals:
Amount of defect fill;
The difference in amount of bone level was calculated by standardized radiographic means using computer aided analysis.
Preparation of PRF
Approximately 10 mm of whole blood was drawn by venipuncture of the antecubital vein and was collected into two blood collection tubes without anticoagulant for PRF preparation. The tubes were immediately centrifuged at 400 g for 10 min at 3000 revolutions per minute at room temperature [14]. The resultant PRF clots were compressed between two sterile gauze pieces and the resultant was used as the membrane.
Surgical Procedure
The intrabony defects were randomly assigned to either control group (bioactive glass putty alone) and test group (bioactive glass putty and PRF) by draw of chits.
Full thickness mucoperiosteal flap was raised and thorough open flap debridement was done under local anaesthesia in the test site the graft (Novabone Putty) was then carefully compacted from the base of the defect coronally. PRF membrane was placed at the test site and was secured with the help of vicryl sutures (Ethicon * Johnson and Johnson). The control site was packed with the graft alone. Non eugenol coe pack was used as dressing.
Postoperatively antibiotic coverage was provided by 500 mg amoxicillin, four times per day; for five days. Analgesic action was provided by ibuprofen thrice daily for three days. Periodontal dressing and sutures were removed two weeks postoperatively.
Statistical Analysis
The results for each clinical and radiographic parameter at each time interval were averaged (mean±SD) and analysed using the independent t-test to establish differences between baseline and postoperative measurements between groups. The difference between each pair of measurements was calculated at baseline, 3, 6 and 9 months postoperatively. Comparisons were made within each group between baseline, 3 months, 6 months and 9 months using the ANOVA test followed by Bonferroni test. All statistical tests were two sided and performed at a significant level of p=0.05.
Results
The mean probing depth reduction was greater in the test group (bioactive glass putty and PRF) i.e., (3.2±2.3 mm) than in the control group (bioactive glass putty alone) i.e., (3.15±1.06 mm). The mean CAL gain was also greater in the test group (4.1±1.73mm)as compared to the control group (3.15±1.06 mm), (p-value< 0.95). Furthermore, significantly greater mean bone fill was found in the test group (7.1 ±1.37 mm) as compared to the control group (5.7±1.64 mm), (p-value<0.043).
Ten pairs of intrabony defects (two sites/subject) were treated with either novabone putty or novabone putty with PRF, on contra lateral site. All treated sites resulted in uneventful healing. All parameters were assessed at baseline, 3 months, 6 months and 9 months. The plaque and gingival indices were assessed [Table/Fig-1a,b]. Reduction in PPD was observed in both, test and control groups from baseline to 9 months (1.65±2.21, 3.20±2.30), (2.10±0.84, 3.15 ±1.06). However, the difference in PPD between the test and the control group was not significant [Table/Fig-2a,b]. A greater gain in CAL from baseline to nine months was found in test group (4.10±1.73) when compared to the control group (3.15±1.06) [Table/Fig-3a,b].
Mean score of plaque index at different intervals.
| Parameters | Full mouth |
|---|
| PI | Mean | S.D. | F-value | p-value |
|---|
| Baseline | 2.74 | 0.31 | 65.458 | <0.001 |
| At 3 months | 1.68 | 0.27 |
| At 6 months | 1.46 | 0.23 |
| At 9 months | 1.35 | 0.16 |
Mean score of plaque index at different intervals.
| Parameters | Full mouth |
|---|
| GI | Mean | S.D. | F-value | p-value |
|---|
| Baseline | 2.78 | 0.15 | 194.703 | <0.001 |
| At 3 months | 1.60 | 0.19 |
| At 6 months | 1.38 | 0.18 |
| At 9 months | 1.28 | 0.10 |
Change in pocket depth among control group and test group at different interval.
| Parameters | Test Group (PRF+Graft) | Control Group (Graft only) | |
|---|
| PD (mm) | Mean | S.D. | F-value | p-value | Mean | S.D. | F-value | p-value |
|---|
| Baseline | 6.00 | 0.94 | 90.332 | <0.001 | 5.90 | 1.10 | 54.591 | <0.001 |
| At 3 months | 2.95 | 0.44 | 3.35 | 0.34 |
| At 6 months | 2.15 | 0.34 | 2.90 | 0.32 |
| At 9 months | 2.40 | 0.46 | 3.00 | 0.24 |
Comparison of mean probing pocket depth reduction (in mm) between control group and the test group at different time intervals using unpaired t-test.
| Parameters | Groups | Mean | S.D. | p-value | Mean Difference |
|---|
| Difference from Baseline to 3 months - PD (mm) | Test Group (PRF+Graft) | 1.65 | 2.21 | 0.169 | -0.45 |
| Control Group (Graft Only) | 2.10 | 0.84 |
| Difference from Baseline to 6 months - PD (mm) | Test Group (PRF+Graft) | 2.60 | 2.37 | 0.9512 | -0.05 |
| Control Group (Graft Only) | 2.65 | 0.82 |
| Difference from Baseline to 9 months - PD (mm) | Test Group (PRF+Graft) | 3.20 | 2.30 | 0.117 | 0.05 |
| Control Group (Graft Only) | 3.15 | 1.06 |
Mean clinical attachment level among control group and test group at different intervals.
| CAL (mm) | Test Group (PRF+Graft) | Control Group (Graft only) | p-value |
|---|
| Mean | S.D. | F-value | p-value | Mean | S.D. | F-value |
|---|
| Baseline | 4.90 | 2.56 | 11.546 | <0.001 | 5.10 | 1.60 | 9.608 | <0.001 |
| At 3 months | 2.35 | 1.45 | 3.00 | 1.22 |
| At 6 months | 1.40 | 1.26 | 2.45 | 1.50 |
| At 9 months | 0.80 | 1.03 | 1.95 | 1.30 |
Comparision of mean CAL gain (in mm) between control and test group at different time intervals using unpaired t-test.
| Parameters | Groups | Mean | S.D. | p-value | Mean Difference |
|---|
| Difference from Baseline to 3 months - CAL (mm) | Test Group (PRF+Graft) | 2.55 | 1.30 | 0.371 | 0.450 |
| Control Group (Graft Only) | 2.10 | 0.84 |
| Difference from Baseline to 6 months - CAL (mm) | Test Group (PRF+Graft) | 3.50 | 1.58 | 0.148 | 0.850 |
| Control Group (Graft Only) | 2.65 | 0.82 |
| Difference from Baseline to 9 months - CAL (mm) | Test Group (PRF+Graft) | 4.10 | 1.73 | 0.155 | 0.950 |
| Control Group (Graft Only) | 3.15 | 1.06 |
The radiographic bone fill from baseline to three months at the control site was observed to be 3.40±1.51 mm, and by the end of 9 months it was 5.70±1.64. At the test site, the mean radiographic bone fill at 3 months was 3.80±0.78 mm and at the end of 9 months was 7.10 ± 1.37 [Table/Fig-4a,b].
Comparision of mean defect fill (in mm) between control group and test group.
| Parameters | Groups | N | Mean | S.D. | p-value |
|---|
| Difference from Baseline to 3 months | Control Group (Graft Only) | 10 | 3.40 | 1.51 | 0.466 |
| Test Group (PRF+Graft) | 10 | 3.80 | 0.78 |
| Difference from Baseline to 6 months | Control Group (Graft Only) | 10 | 3.50 | 1.43 | 0.049* |
| Test Group (PRF+Graft) | 10 | 4.80 | 1.32 |
| Difference from Baseline to 9 months | Control Group (Graft Only) | 10 | 5.70 | 1.64 | 0.043* |
| Test Group (PRF+Graft) | 10 | 7.10 | 1.37 |
Comparison of mean defect fill (in mm) between control group and test group using unpaired t-test.
| parameters | Groups | N | Mean | S.D. | p-value | Mean difference |
|---|
| Difference from Baseline to 3 months | Control Group (Graft only) | 10 | 3.40 | 1.51 | 0.466 | -0.40 |
| Test Group (PRF+Graft) | 10 | 3.80 | 0.78 |
| Difference from Baseline to 6 months | Control Group (Graft only) | 10 | 3.50 | 1.43 | 0.049* | -0.70 |
| Test Group (PRF+Graft) | 10 | 4.80 | 1.32 |
| Difference from Baseline to 9 months | Test Group (PRF+Graft) | 10 | 7.10 | 1.37 | 0.043* | 1.40 |
| Control Group (Graft only) | 10 | 5.70 | 1.64 |
Discussion
The primary goal of periodontal therapy is to arrest the progression of periodontal disease and also regeneration of the lost periodontal tissues. Periodontal regeneration is a complicated process involving a number of different cell types and cell stromal interactions for complete regeneration. Therefore, for periodontal regeneration of intraosseous defects, root-conditioning agents, guided tissue regeneration procedures, bone replacement grafts and growth attachments factors or combination of these materials have been used with various degree of success [15].
In the recent past, bioactive glass, a synthetic biocompatible alloplastic material that possesses osteo-conductive and osteo-stimulative properties has been used as bone replacement graft for periodontal osseous defects [16].
PRF is a second-generation platelet concentrate which contains platelet and growth factors, has physical and biochemical attributes that make it attractive for application in periodontal wound healing, and for these reasons it was investigated as a potential regenerative agent for intrabony periodontal defects. The present study was conducted to evaluate the regenerative potential of combining PRF with bioactive glass putty and comparing when bioactive glass putty is used without addition of PRF clinically and radiographically.
There was a statistically significant improvement in the plaque and gingival index scores in both the groups indicating reduction in inflammation, positive patient motivation from baseline to 9 months. Significant reduction in probing pocket depth, gain in CAL and radiographic bone level was found in both the groups when compared from baseline to nine months postoperatively, which is similar to earlier studies reported in the literature [15-20].
Studies in the past have focused on PRP combined with bovine porous bone mineral or other alloplasts [21-25] and studies on PRF combined with bovine porous bone material [26] and demineralized freeze-dried bone allograft [27]. Recently, a comparative evaluation of bioactive glass and platelet rich fibrin has been reported in the treatment of furcation defects [16].
In the present study, a greater gain in clinical attachment level from baseline [Table/Fig-3a,b] to 9 months [Table/Fig-5] in test group as compared to control group was observed. The difference in pocket depth reduction and clinical attachment gain across the groups were not statistically significant. A marked gain in the radiographic bone fill was noted at both control [Table/Fig-6] and the test sites [Table/Fig-7] and the difference was statistically significant from baseline to 9 months. Comparison across the groups also showed the results to be statistically significant at the end of 3, 6 and 9 months [Table/Fig-4a,b]. In the present study, PRF seems to have an additional favorable effect on defect fill in the treatment of periodontal intrabony defects.
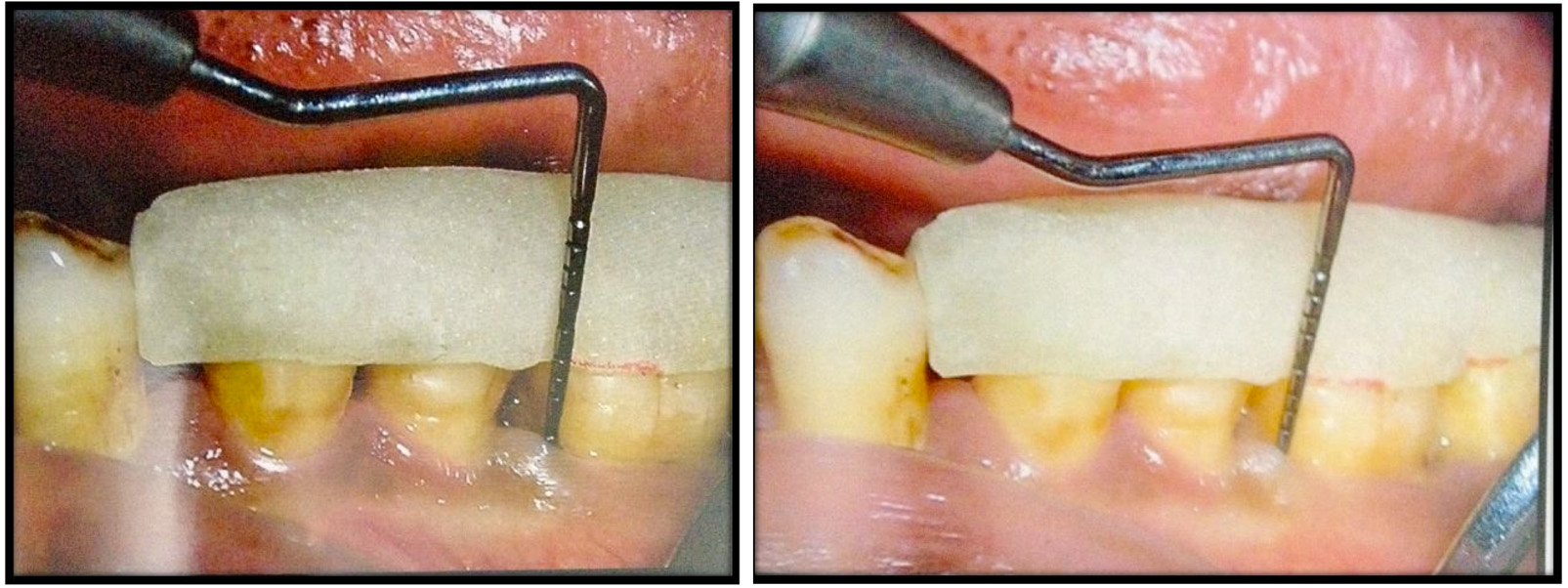
a) CAL measurement at the baseline (Test Group); b) CAL measurement at 9 months postoperatively (Test Group).
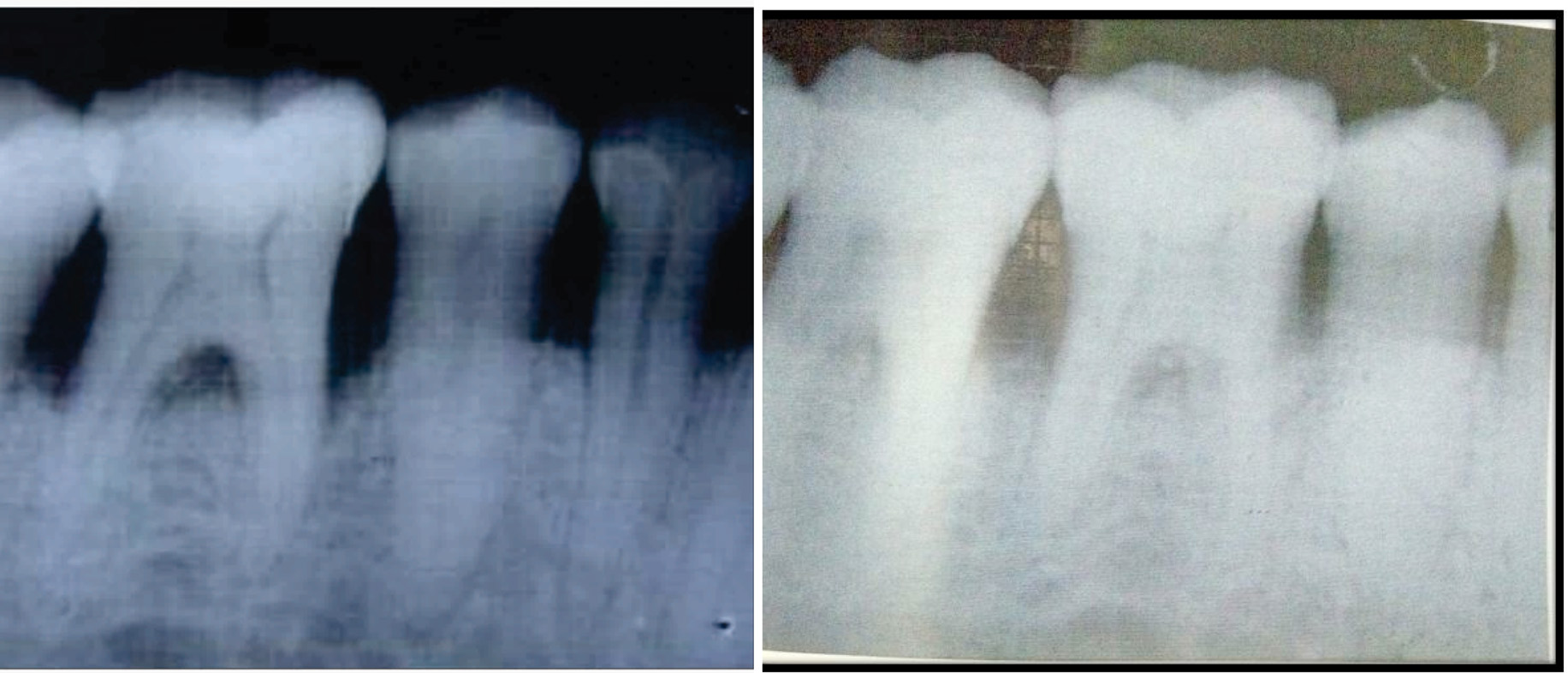
a) Radiograph showing the bone fill at 9 months postoperatively (Control Group); b) Radiograph showing the bone fill at 9 months postoperatively (control Group).
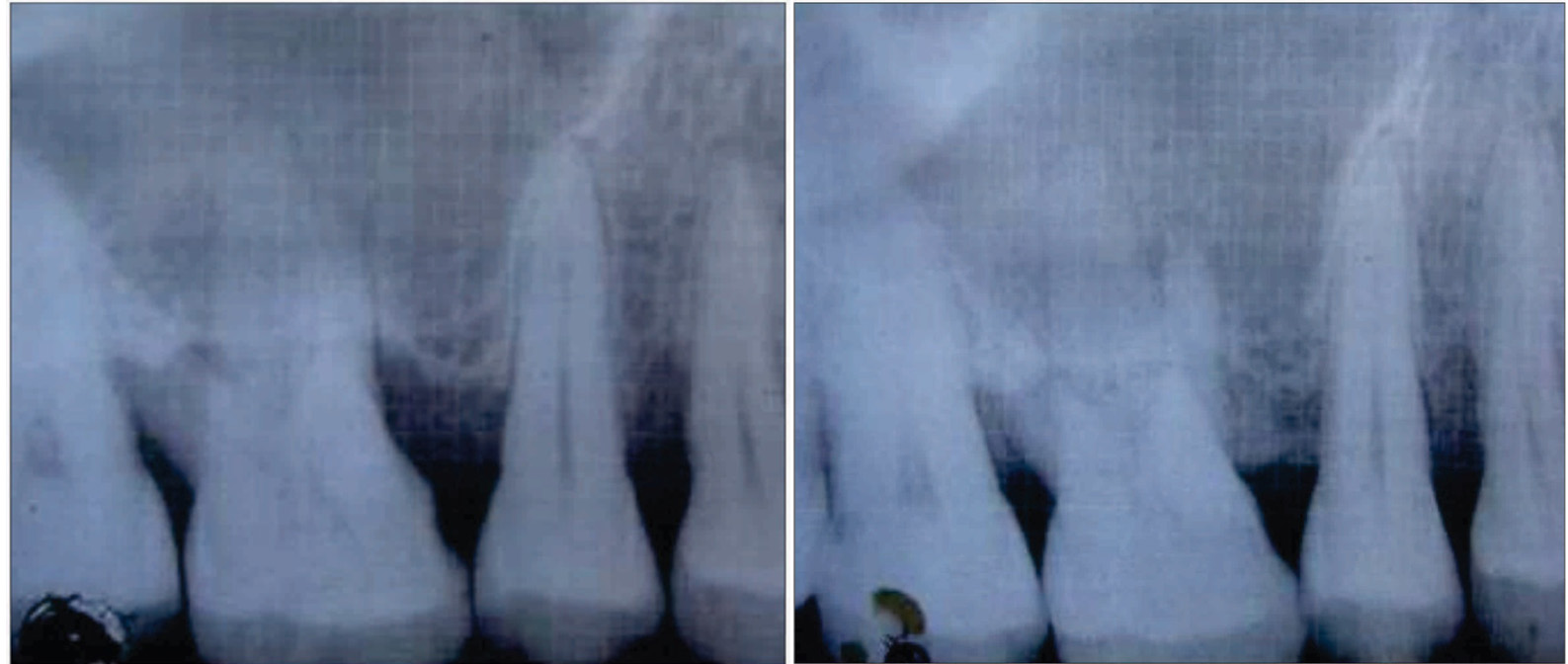
a) Radiograph showing the bone fill at baseline (Test Group); b) Radiograph showing the bone fill at 9 months postoperatively (Test Group).
This result may be attributed to beneficial effects of PRF as it contains a dense fibrin matrix. Moreover, PRF takes longer to be resorbed by the host, which leads to a slow and sustained release of platelet and leukocyte derived growth factors into the wound area where they are required [26]. As PRF contians leukocytes, it can exert an antibacterial effect in the wound. Last but not the least; it is a great source of vascular endothelial growth factor, which has key role in angiogenesis [26].
Many in vitro analysis have also shown a beneficial effect of PRF like its effect on proliferation and differentiation on osteoblasts leading to enhanced bone healing [10].
PRF fragments serve as biological connector between bone particles. The release of cytokines from the PRF plays a significant role in the self-regulation of inflammatory and infectious phenomenon with the grafted material. Thus, based on the above facts, PRF is simpler and cost-effective to prepare, moreover, it is safer for the patients as it does not expose them to animal-derived anticlotting agents as required for the preparation of PRP [26].
Study by Lekovic V et al., showed greater defect fill on buccal and lingual sites in sites treated with PRF-BPBM [26]. The leukocytes play a role in the release of growth factors, regulation of immune cells, anti-infectious activity, and matrix remodeling in the healing phase. It is an optimal matrix for migration of endothelial cells and fibroblasts [27]. Treatment of periodontal intrabony osseous defects with bioactive glass putty alone as well as in combination with PRF demonstrated radiographic osseous defect fill. Bioactive glass when in contact with the body fluids undergoes an ionic exchange; the cations are leached from the surface in exchange for hydronium or hydrogen ions forming silanol groups (SiOH). This ionic exchange increases the interfacial pH [28]. Silanol group through a polycondensation reaction form a silica rich gel layer on the particulate surface. This layer having a high surface area results in production of a site for the re-deposition of calcium and phosphorous from the graft material as well as the blood [29]. With time as the thin calcium phosphorous layer builds up in thickness and a crystalline Hydroxycarbonate Apatite (HCA) layer identical to bone material is formed; bonding of bone and this material occurs through this apatite layer. It is hypothesized that PRF in the test site provides additional benefit, through delivery of growth factors as an adjunct to bone graft. The growth factors in PRF are active for a longer period as the natural fibrin framework of PRF protects growth factors from proteolysis [30]. Thus, we observed a positive clinical impact in additional application of PRF membrane with bioactive glass putty in treatment of periodontal intrabony defect. However, bone fill data derived from surgical re-entry are important to substantiate routine postoperative measurement data. In addition, histological evaluation of the treated periodontal intrabony defect sites is the only dependable method to establish the nature of interface at the periodontal soft and hard tissue. This therefore can be considered as a limitation of our study. Use of PRF as an adjunct to bone grafts is a viable option in bone regenerative techniques. Also the next generations of PRF e.g., injectible PRF should also be explored for potential bone regeneration. Further studies evaluating bone regeneration by surgical re-entry should be planned. Studies on a larger sample size with a longer follow up should be designed.
Conclusion
Within the limits of the present study, it can be concluded that the combination of bioactive glass and platelet-rich fibrin membrane was effective in improving the radiographic parameters along with soft tissue parameters, enhancing the clinical outcome of the therapy compared to the bioactive glass putty alone.
The present study showed significant reduction in plaque index, gingival index and probing pocket depth in both the groups at different time intervals after the periodontal therapy.
The following conclusions were drawn from the present study:
There was significant reduction in plaque index, gingival index and probing pocket depth in both the groups at different time intervals after the periodontal therapy.
There was significant gain in the clinical attachment level in both the groups.
Radiographically, there was significant defect fill, in both groups.
Both bioactive glass putty and PRF were safe to use, without causing any immunologic/antigenic reactions in any of the treated patients.
Both groups showed the potential of enhancing the periodontal regeneration.
The bioactive glass putty appears to be a suitable vehicle to administer biologic substances like PRF and growth factors to induce the new bone regeneration.
The addition of PRF to bioactive glass putty improved the handling characteristics of bioactive glass putty. Easy placement and stabilization of the graft at the defect site, were the additional clinical advantages.