Psoriasis is a persistent chronic relapsing inflammatory skin disorder. It is considered as a hyper proliferative disease with immune-mediated basis and genetic predisposition. About 2%-3% of the population is affected by psoriasis. The lesions usually consist of red plaques with scales, sharp demarcation and indurations. Most lesions are present over extensor surfaces and scalp. The disease is variable in extent, duration and periodicity of flares [1].
Histological features of psoriatic skin lesions include acanthosis, parakeratosis, diminished to absent granular cell layer, presence of Munro’s microabscess, elongation and oedema of dermal papillae, dilated and tortuous capillaries as well as marked infiltration of mononuclear cells into dermis [2].
Although, the triggering events of psoriasis are still unknown, many environmental factors have been shown to play a role. Physical agents such as infection, stress, drug and alcohol can be an external trigger in genetically predisposed individuals [3]. A complex role for T-lymphocytes and a variety of cytokines is proven in psoriasis [4].
Apoptosis is an energy dependant programmed cell death that appears to be the mode by which damaged cells are removed from the lesional tissue in psoriasis. Caspase-3 is an enzyme that plays a key role in apoptosis; it is a member of the family of cysteinyle aspartate specific proteases. Caspase-3 has been called the executioner caspase “henchman that goes around and executes the cell”. CPP32, apoptin or YAMA are its other synonyms. Caspase-3 exists as an inactive proenzyme which undergoes proteolytic processing and dimerizes to the active enzyme form [5,6]. In the current study, we aimed to evaluate the expression of caspase-3 in Egyptian psoriasis patients. Another aim was to correlate this expression with the clinicopathological parameters in order to identify the possible hypothesized role of caspase-3 in the pathogenesis of psoriasis.
Materials and Methods
Ethics: A written consent approved by The Local Ethical Research Committee in Faculty of Medicine Menoufia University was obtained from every subject before the study initiation. This was also in accordance with the Helsinki Declaration of 1975 (revised in 2000) [7].
Studied Population: This case-control study included 20 patients presenting with chronic plaque psoriasis and 10 controls. They were selected from outpatient clinic of dermatology, Menoufia University Hospital in the period between October 2014 and January 2016. Normal skin samples were obtained from subjects attending plastic surgery department. Biopsies from cases and control subjects were site-matched.
Clinical data and variables including patients’ demographics (age and gender) as well as (site, size of lesions and duration of disease) were obtained and documented.
Exclusion criteria included other chronic inflammatory skin disease, pustule or erythrodermic psoriasis. In addition, patients who had received any topical or systemic therapy for at least four months were excluded.
Histopathological examination of Haematoxylin and Eosin (H&E)-stained sections for confirmation of the diagnosis of psoriasis was done.
Immunohistochemical (IHC) staining for caspase-3: Nearly, 4 μm-thick sections were cut from the paraffin-embedded blocks and stained by IHC technique according to data sheet (Cat. #RP-096-05) (Diagnostic Biosystems, 6616 Owens drive Pleasanton, CA, 94588). The slides were incubated overnight at room temperature with a purified rabbit polyclonal antibody raised against caspase-3. It is received as 0.5 ml concentrated for use. The Envision (Dako, Glostrup, Denmark) method was used for detection of antibody binding. The reaction was visualized by an appropriate substrate/chromogen (diaminobenzidine) reagent with Mayer’s haematoxylin as a counter stain.
Interpretation of IHC results: Immunohistochemically, caspase-3 expression was confirmed by cytoplasmic and/or nuclear stain in examined cells [8].
For every antibody, the following items were assessed:
The epidermis of normal skin biopsies, and psoriasis were assessed for
Expression: positive or negative;
Percentage of positive cells: assessed at 20X magnification [9];
Intensity: Intensity of expression categorized into two groups mild-moderate and strong
Histo-score (H score): H score was calculated according to equation: H score = 1 x % of mildly stained cells + 2 x % moderately stained cells + 3 x % of strongly stained cells [10];
Distribution: patchy or diffuse;
Pattern: cytoplasmic or nucleo-cytoplasmic;
Type of the stained epidermal cells: basal or basal and suprabasal.
The dermis of normal skin biopsies and psoriasis were assessed for the following:
Expression: positive or negative;
Type of stained dermal cells: inflammatory or adnexa.
Statistical Analysis
Data were collected, tabulated and statistically analysed using a personal computer with “(SPSS) version 12” program (SPSS Inc, Chicago, Illinois, USA). Values were expressed in number, percentage and mean±standard deviation (X±SD) when appropriate. Fisher’s-Exact test was used to evaluate exact probabilities in a contingency table (usually 2x2) when the expected cell count of more than 25% of cases was less than five. Chi-square (χ2) was used to study the association between two qualitative normally distributed variables. Mann-Whitney U test was used for comparison between two groups not normally distributed having quantitative variables. Spearman’s coefficient was used to measure the correlation between two quantitative variables. Kruskal Wallis test was also used. Differences were considered statistically significant with p<0.05 and highly significant when p-value <0.001.
Results
There were 10 male and 10 female between 18 to 56 years of age. The mean age of the study population was found to be 37.85±10.51 years. Ten apparently healthy individuals and without family history of psoriasis representing the control group were age and sex matched. They included six males and four females with their ages ranging between 21 and 56 years and a mean±SD with the mean age of 41.60±10.37.
The age of onset of disease varied in the studied cases and ranged from 12 to 40 years a mean±SD (29.0±9.24). Duration of disease varied in the studied cases and ranged from two to 20 years with a mean±SD of 8.85±5.61 years. Family history was positive in four cases (20%) and negative in 16 cases (80%).
Regarding the triggering factors of psoriasis, trauma was found in 8 cases (40%), stress was found in 8 cases (40%) while drugs were only found in four psoriatic cases (20%).
Most of cases (70%) presented with early onset type of psoriasis and only six cases (30%) were of late onset type. Nine patients (45%) presented with lesions in the trunk (including face and trunk), 10 (50%) patients presented with lesions in the extremities, while only one case (5%) presented with lesions in both the trunk and the extremities.
Scalp was affected in four cases (20%), palm and sole were affected in four cases (20%), nail in six cases (30%), joint in five cases (25%) and mucosa was affected in three cases (15%). Itching was the main complaint in 14 cases (70%) and was not recorded in other patients. Koebnerization was detected in 8 cases (40%) and was absent in 12 cases (60%). Psoriasis Activity Severity Index (PASI) for the studied cases ranged from 25 to 47 with a mean±SD 38.53 ±6.12.
IHC Expression of Caspase-3 in Studied Groups
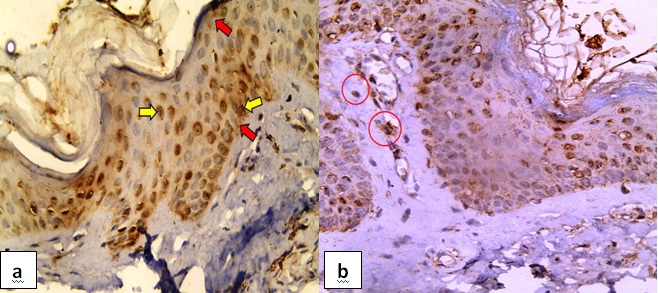
Normal skin: All the cases expressed caspase-3; half of them were diffusely distributed while the rest were of patchy distribution. Sixty percent of the cases displayed mild to moderate nucleo-cytoplasmic expression in the lower 2/3 of the epidermal layer. H score ranged from 30 to 270 with a mean value of 109. All the cases expressed caspase-3 in the inflammatory cells and 70% in the adnexa [Table/Fig-1].
Caspase-3 in normal skin- a) Nuclear (yellow arrows) and cytoplasmic expression (red arrows) in the covering epidermis (Immunoperoxidase, 40X); b) Caspase expression in the perivascular inflammatory infiltrates (red circles) (Immunoperoxidase, 40X).
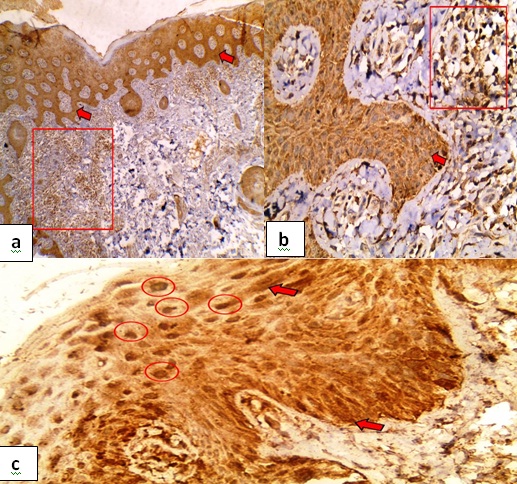
Psoriasis: Caspase-3 was expressed in 85% of the cases. Diffuse, strong and nucleo-cytoplasmic expression was noticed in 82.4%, 70.6% and 88.2% cases respectively. H score ranged from 70-240. Ninety five percent of cases expressed caspase-3 in the inflammatory infiltrates while none of the adnexa expressed caspase-3 [Table/Fig-2].
Caspase-3 in psoriasis- a) diffuse nucleo-cytoplasmic expression in the overlying epidermis (red arrows), caspase-3 was also expressed in the sub-epithelial inflammatory infiltrate (red box) (Immunoperoxidase, 4X); b) Higher power of the previous slide exhibiting diffuse cytoplasmic expression in the overlying epidermis (red arrow) and its expression in the subepithelial inflammatory infiltrate (red box). (Immunoperoxidase, 40X); c) Diffuse cytoplasmic expression in the overlying epidermis (red arrow) and its expression in the nucleus (red circle) (Immunoperoxidase, 40X).
Psoriasis vs. control cases: High caspase-3 H score was signifi-cantly in favour of psoriatic group in comparison with the control group (p=0.03). On the contrary, in the dermis, caspase-3 was significantly higher in skin adnexa while completely absent in the psoriatic group (p=0.03) [Table/Fig-3].
Comparison between psoriasis and control group regarding Caspase-3 immunohistochemical profile.
| Caspase-3 (epidermis) | Studied groups | Test | P-value |
|---|
| Cases N = 20 | Controls N = 10 |
|---|
| No | % | No | % |
|---|
| ExpressionPositiveNegative | 173 | 8515 | 100.0 | 1000.0 | FE1.67 | 0.53 |
| IntensityMild – moderateStrong | 512 | 29.470.6 | 64 | 60.040.0 | FE2.44 | 0.22 |
| DistributionPatchyDiffuse | 314 | 17.682.4 | 55 | 5050 | FE3.16 | 0.10 |
| Cellular localizationCytoplasmicNucleocytoplasmic | 215 | 11.888.2 | 46 | 4060 | FE2.90 | 0.15 |
| Type of cellBasal cellsSuprabasal cellsAll | 197 | 5.952.941.2 | 262 | 206020 | 2.03 | 0.36 |
| PercentX ±SDRange | 66.47±19.7430 – 100 | 47.0±18.8930 – 80 | U2.13 | 0.03* |
| H scoreX ±SDRange | 156.47±67.6370 – 240 | 109.0±74.330 – 270 | U1.72 | 0.09 |
| Caspase-3 (dermis) | |
| Inflammatory cellsPositiveNegative | 191 | 95.05.0 | 100 | 1000.0 | FE0.52 | 1.0 |
| Adnexal cellsPositiveNegative | 020 | 0100 | 37 | 3070 | FE6.67 | 0.03* |
X = Mean, SD= Standard deviation, FE = Fisher’s-Exact test, U = Mann-Whitney U test
Association between intensity of caspase-3 expression in psoriasis and clinicopathological parameters: Strong caspase-3 expression was significantly in favour of high PASI score (p=0.005), early onset lesions (p=0.01) and lesions in the extremities (p=0.03). However, no significant relation was found with other clinicopathological parameters [Table/Fig-4].
Association between caspase-3 intensity and clinicopathological data.
| Parameter | Intensity among cases | Test U | p-value |
|---|
| Mild and moderate N = 5 | Strong N = 12 |
|---|
| Age/yearX ±SDRange | 44.80±10.9927 – 56 | 36.18±9.2222 – 52 | 1.69 | 0.09 |
| Age of onsetX ±SDRange | 33.4±12.1612 – 40 | 28.75±6.84 18 – 37 | 1.59 | 0.11 |
| Duration of the diseaseX ±SDRange | 11.40±5.683 – 16 | 7.42±4.912 – 20 | 1.38 | 0.17 |
| PASI ScoreX ±SDRange | 31.80±6.0625 – 37 | 41.33±3.4736 – 47 | U2.81 | 0.005* |
| n | % | n | % | | |
| SexMaleFemale | 14 | 2080 | 84 | 66.733.3 | 3.09 | 0.13 |
| Type of psoriasisEarly onsetLate onset | 14 | 2080 | 111 | 91.78.3 | 8.73 | 0.01 |
| Family historyPositiveNegative | 50 | 1000.0 | 93 | 75.025.0 | 1.52 | 0.52 |
| SiteTrunkExtremitiesTrunk & Extremities | 401 | 800.020 | 480 | 33.366.70.0 | 7.37 | 0.03* |
| ScalpAbsentPresent | 50 | 1000.0 | 84 | 66.733.3 | 2.18 | 0.26 |
| Palm & soleAbsentPresent | 50 | 1000.0 | 84 | 66.733.3 | 2.18 | 0.26 |
| NailAbsentPresent | 23 | 4060 | 102 | 83.316.7 | 3.19 | 0.12 |
| JointAbsentPresent | 50 | 1000.0 | 84 | 66.733.3 | 2.18 | 0.26 |
| MucosaAbsentPresent | 50 | 1000.0 | 93 | 75.025.0 | 1.52 | 0.52 |
| ItchingAbsentPresent | 32 | 6040 | 39 | 2575 | 1.89 | 0.28 |
| KoebnerizationAbsentPresent | 32 | 6040 | 66 | 5050 | 0.14 | 1.0 |
| Triggering factorsStressTraumaDrugs | 140 | 20800.0 | 444 | 33.333.333.3 | 3.51 | 0.17 |
X = Mean, SD= Standard deviation, U = Mann-Whitney U test, χ2 = Chi-squared test, FE = Fisher’s-Exact test
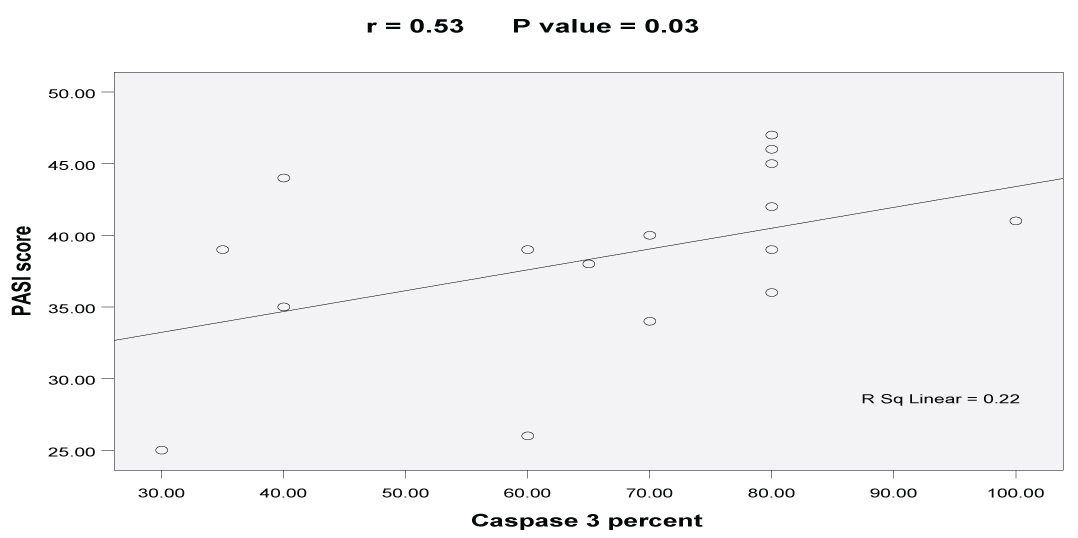
Correlation between caspase-3 expression in psoriasis and the studied clinicopathological parameters: Significant positive correlation was found between caspase-3 percent and PASI score (r= +0.53, p-value=0.03). Lower correlation coefficient of age, age of onset and duration was detected with no significant difference in caspase-3 expressions [Table/Fig-5,6].
Correlation between Caspase-3 percent and clinical data.
| Parameters | CASPASE- 3 percent |
|---|
| R | p-value |
|---|
| Age | - 0.09 | 0.66 |
| Age of onset | -0.06 | 0.83 |
| Duration | 0.07 | 0.80 |
| PASI score | 0.53 | 0.03* |
r= correlation coefficient
Significant positive correlation between Caspase-3 percent and PASI score.
Caspase-3 H score in psoriasis and clinicopathological parameters: High caspase-3 H score was in favour of early onset psoriasis (p-value =0.009) [Table/Fig-7].
Caspase-3 H score in psoriasis and clinical data.
| Parameters | Caspase-3 H score | Test | P-Value |
|---|
| X ±SD | Range |
|---|
| SexMaleFemale | 74.4±61.7136.3±72.3 | 90 – 24070 – 240 | U 1.37 | 0.17 |
| Type of psoriasisEarly onsetLate onset | 184.2±61.690.0±7.1 | 70 – 24080 – 100 | U 2.63 | 0.009* |
| Family historyPositiveNegative | 140.7±63.8230.0±17.3 | 70 – 240210 - 240 | U 1.92 | 0.06 |
| SiteTrunkExtremitiesTrunk and Extremities | 136.3±64.6186.3±63.080.0 | 70 – 240100 – 240 | K5.03 | 0.08 |
U = Mann-Whitney U test, K = Kruskal Wallis test
Discussion
Psoriasis is a common skin disease characterized by accelerated turnover and incomplete differentiation of epidermal keratinocytes as well as by inflammatory infiltrates of T lymphocytes in dermis and epidermis. It is recognized as a T cell mediated disease resulting from aberrant activation of both innate and adaptive immunity [11,12].
Apoptosis is a programmed cell death. Impairment in apoptosis can elicit neoplastic or autoimmune diseases. Psoriasis is recognized as the most widespread autoimmune disease [13,14]. Caspase-3 is a marker of apoptosis execution entry point and apoptosis plays an important role in pathogenesis of psoriasis [15,16].
The expression of caspase-3 in psoriasis and its relation with the available clinicopathologic parameters together with its expression in the normal skin was studied in the current work.
In the current study, caspase-3 was expressed in all studied cases of normal skin. Our results are in concordance with Raymond AA et al., who stated that caspase-3 displayed clear expression in the epidermis which may be involved in keratinocyte differentiation, and may induce apoptosis in response to exposed cutaneous damage [17]. These findings show that activation of caspases does not necessarily indicate that the cell is programmed to die. In fact, a low level of caspase activation seems to play a crucial role in normal skin physiology. Non apoptotic role of caspase-3 has been recently evident [18].
As for the expression of caspase-3 in the studied psoriasis cases, 85% expressed caspase-3; about 71% were of strong expression. Caspase-3 in the psoriatic cases was mainly nucleo-cytoplasmic and detected more frequent in the lower 2/3 of epithelial layers.
Regarding the nucleo-cytoplasmic expression of caspase-3 in the lower 2/3 of epithelial layers of the psoriatic cases, this goes in concordance with the results of Tobón-Arroyave SI et al., Bascones-Ilundain C et al., and Abdel-Latif AM et al., who worked on lichen planus. They reported that caspase-3 is expressed more often in basal and supra-basal layers and in both cytoplasm and nuclei [19-21].
As for the staining intensity, prevalence of strong caspase-3 expression reported in this study is in agreement with Tobón-Arroyave SI et al., who reported strong staining of caspase-3 especially in basal layer [19]. On the contrary, Bascones-Ilundain C et al., and Abdel-Latif AM with her colleagues reported more frequent mild staining of epithelial cells. Abdel-Latif AM et al., found weak intensity of caspase-3 with a diffuse distribution in basal and suprabasal layers in 10% of controls including both oral and cutaneous lesions [20,21].
In the current study, strong caspase-3 expression is in favour of early onset psoriatic lesions located in the extremities. In addition to strong caspase-3 intensity, H score was in favour of early onset psoriasis. According to García-Diez A et al., the irregular course that tends to be more aggressive and common to be generalized has been linked to early onset psoriasis [22]. This also could link higher percentage and strong expression of caspase-3 with clinically severe cases. Chen J et al., found that activated caspase-3 was significantly higher in the severe and progressive stage psoriasis lesions [23].
As for the relation between the strong intensity and the presence of psoriasis in the extremities, it can be attributed to the exposure of the extremities to the sun more than the trunk. Sun exposure causes cutaneous damage which may stimulate intense caspase-3 expression that could induce apoptosis as previously stated by Raymond AA et al., [17].
PASI score was developed in 1978 to assess the effects of retinoids in psoriasis [24]. The affected area and lesion characteristics are entered in a formula that results in a score from 0 to72 [25]. PASI score is often used as the standard criteria in the validation of new measures and correlated well in most cases with other physician-based assessment [26].
In the present study, significantly higher PASI score was detected among cases with strong caspase-3 intensity (p-value=0.005). Significant positive correlation was found between caspase-3 H score and PASI score (r=+0.51, p-value = 0.03). This could indicate that higher expression and increased intensity of caspase-3 goes hand in hand with the severity of the lesions and the progressed clinical picture. Our results differ from Chen J et al., which stated absence of any correlations between PASI and the expression of activated caspase-3 in psoriasis vulgaris lesions [23].
In the current study, high H-score was significantly in favour of psoriasis in comparison with the normal skin biopsies. Our results are in agreement with a study done on caspase 5, which stated up regulation of caspase 5 in psoriatic lesional skin in comparison with the non lesional cases, suggesting an important role in the inflammatory response in psoriasis [27].
In the dermis, caspase-3 expression was in favour of the normal control group. This could indicate that adnexal cells do not play a major role in the immune mediated pathogenesis of psoriasis.
Limitation
Funding was the main limitation in the current study. We aim for further analysis of the molecular basis of pathogenic and prognostic role of caspase-3 in psoriasis.
Conclusion
The aforementioned findings may shed light on the anti-apoptotic physiologic role of caspase-3 in normal skin. Caspase-3 over expression in the psoriatic lesion proposes a potential role in the pathogenesis of psoriasis. Positive correlation between the caspase-3 expression and the early onset psoriatic lesion located in the extremities implies a possible poor prognostic impact of caspase-3 overexpression. Further studies regarding the possible role of anti caspase-3 targeted therapy of psoriasis are recommended.