Nitrofurantoin (NF) is an age old drug, has been used for more than 50 years for treatment of uncomplicated UTIs. In comparison to the other classes of antimicrobial agents, acquired resistance to NF is quite infrequent because of its complex mechanism of action [11,12]. The drug uses the flavoproteins of bacterial cell to convert into multiple intermediate forms which damages the DNA. It also inhibits carbohydrate metabolism and interfere with the cell wall synthesis. But, there is very limited data on NF activity and the risk factors associated with VRE in India. Therefore, the following study was carried out to assess the role of NF against VRE isolates from UTI.
Materials and Methods
An observational study over a period of six months (November 2015 to April 2016) was carried out at the Department of Microbiology, All India Institute of Medical Science, New Delhi, India, to find out the in vitro action of NF against VRE isolates isolated from urinary tract infection. All the culture sensitivity testing was done as a part of routine diagnostics during the outdoor visit or indoor stay of the patient for which consent of the patient is not required. The urine samples from the symptomatic patients were processed within two hours of collection on Cystine Lactose Electrolyte-Deficient (CLED) agar. Enterococcus spp. were isolated from urine specimens and identified with standard biochemical tests (growth in media containing 6.5% sodium chloride and bile-esculin hydrolysis).
Colony forming units were counted by using standard semi-quantitative methods and speciation was performed by testing for carbohydrate fermentation (mannitol, arabinose). Consecutive same organism in two or more occasions within the duration of one week in same patient was considered as single isolate. The antibiotic sensitivity pattern was determined using Muller Hilton agar by Kirby-Bauer disc diffusion method as per CLSI 2015 guidelines[13]. The antibiotics (HiMedia, India) tested were Pencillin (10 Units), Ciprofloxacin (5 μg), Nitrofurantoin (300 μg), High level Gentamicin (120 μg), Erythromycin (15 μg), Teicoplanin (30 μg), Vancomycin (30 μg) and Linezolid (30 μg). Standard strains of Staphylococcus aureus ATCC® 25923TM and E. faecalis ATCC® 29212TM were used as controls for the antibiotic susceptibility testing. For the quality control of Mueller Hinton agar to determine the appropriate thymidine content, E. faecalis ATCC® 29212TM with trimethoprim/sulfamethoxazole discs were tested.
Statistical Analysis
The Wilcoxon rank-sum (Mann-Whitney) test was used to compare continuous variables such as age of patients and length of hospital stay. Chi-square or Fisher’s-exact tests were used to compare categorical variables like sex and distribution of inpatients in ICU and wards. The p-value <0.05 was considered significant.
Results
In total 14,714 urine samples were received over a period of six months out of which 942 (6.4%) samples were culture positive. The age of the patients ranged from three months to 88 years with mean age of 42.5 years. Female patients were predominant (54.2%, n=38) in comparison to male (45.7%, n=32). Majority (90%) of the patients were adults (≥ 18-year-old) [Table/Fig-1].
Age and sex distribution of patients positive for Enterococcus spp.
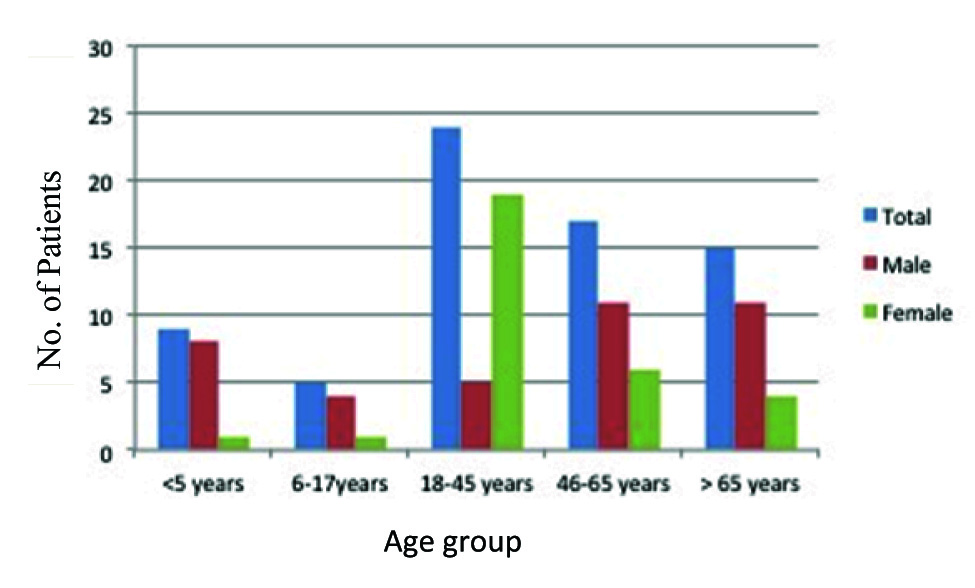
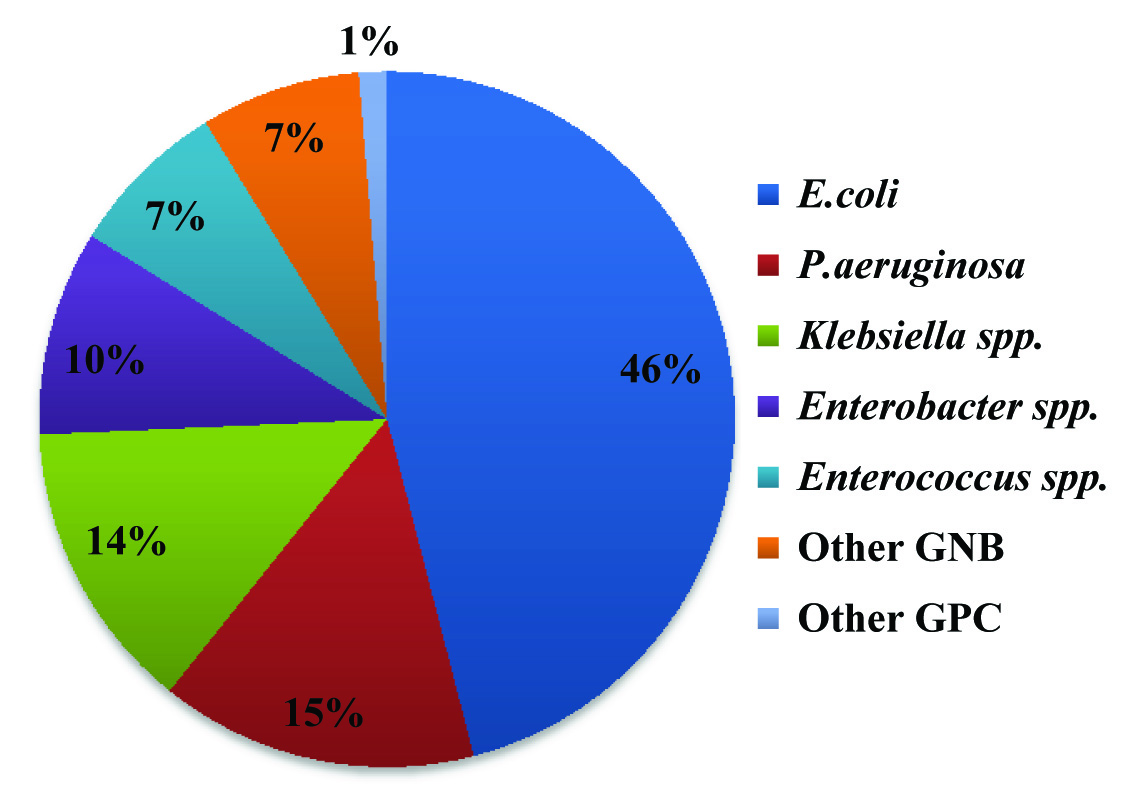
Eighty nine percent of the total samples were received from hospitalised patients. Only 1% (n=16) among the samples from outdoor patient department were found culture positive showing significant bacteriuria. However, all the Enterococcus spp. was isolated from admitted patients only. Gram Negative Bacilli (GNB) were isolated predominantly in comparison to Gram Positive Cocci (GPC). Most common bacterial isolate was Escherechia coli (45.8%) followed by Pseudomonas spp.(14.7%), Klebsiella spp.(13.5%) and Enterobacter spp.(9.5%). Seventy isolates (7.4%) were identified as Enterococcus spp [Table/Fig-2].
Bacterial spectrum of various urinary isolates. Other GNB: Acinetobacter spp. Other GPC: Coagulase negative Staphylococcus
Among the enterococcal isolates, 61 (87%) were identified as E. faecium and 9 (13%) were E. faecalis. Approximately 37% (n=26) of the total Enterococcus spp. were detected as VRE.
The mean age of VRE patients were observed to be higher than Vancomycin Susceptible Enterococci (VSE) by five years.
Prolonged hospitalisation and increased length of stay in intensive care unit were observed to be important risk factors for VRE bacteriuria (p-value<0.05) [Table/Fig-3].
Comparision of demographic profile of patients with VRE and non-VRE.
| Variables | VRE (n=26) | NoN-VRE (n=44) | p-value |
|---|
| Mean age | 48.62 | 38.5 | 0.07 |
| Median age | 55 | 50 |
| Sex |
| Male | 7 | 25 | 0.648 |
| Female | 19 | 19 | 0.548 |
| Urinary Catheter | 22 | 34 | - |
| Mean length of hospital stay | 46.5 | 26.2 | 0.026 |
| Ward | 18 | 40 | - |
| ICU | 8 | 4 | 0.045 |
All the isolates of enterococci were observed susceptible to linezolid (100%) [Table/Fig-4]. However, nitrofurantoin (81.4%) susceptibility also had encouraging results. Among 26 VRE isolates, 21(80.76%) were susceptible to nitrofurantoin. High Level Aminoglycoside Resistance (HLAR) was detected in 81.4% of the isolates. Resistance to vancomycin was observed higher in E. faecium (96%) than E. faecalis (4%) while there was no significant difference between two species for susceptibility towards nitrofurantoin [Table/Fig-5].
Antibiotic susceptibility pattern of Enterococcus species Pencillin (10 U), Ciprofloxacin (5 μg), Nitrofurantoin (300 μg), HLG (High Level Gentamicin) (120 μg), Erythromycin (15 μg), Teicoplanin (30 μg), Vancomycin (30 μg), Linezolid (30 μg).
| Antibiotic | E. faecalis (n=9) | E. faecium (n=61) |
|---|
| Vancomycin | 88% | 59% |
| Nitrofurantoin | 80.32% | 88.8% |
| Ciprofloxacin | 11.4% | 22.2% |
| Teicoplanin | 59.01% | 88.8% |
| Erythromycin | 22.2% | 8.1% |
| Pencillin | 22.2% | 14.7% |
| Linezolid | 100% | 100% |
| HLG | 33.3% | 13.1% |
Comparison of sensitivity rate for vancomycin and nitrofurantoin.
| Parameters | NF resistant isolates | NF sensitive isolates | Total |
|---|
| E. faecium | E. faecalis | E. faecium | E. faecalis |
|---|
| Vancomycin Resistant | 5 | 0 | 20 | 1 | 26 |
| Vancomycin Sensitive | 7 | 1 | 29 | 7 | 44 |
| Total | 12 | 1 | 49 | 6 | 70 |
Nitrofurantoin found to have maximum susceptibility against VRE after linezolid.
Discussion
Enterococcus spp. are one of the most common nosocomial pathogens. It is the second most leading cause of nosocomial UTI and the third most common cause of nosocomial bacteraemia in the United States. The highest rate of Enterococcus spp. causing UTI was reported from Canada (16.8%), followed by the US (12.5%) and Europe (11.7%) [14]. Incidence of UTI due to Enterococcus spp. in India varies from 0.05%-13% among different population [7,15]. Enterococci were isolated from 7% of the patients in the current study which is in concordance with previous study by Mohanty S et al., [16]. However, the distribution of species is observed to be much different. All the enterococcal isolates in the earlier study from the same institute from urine sample were E. faecalis in comparison to a drastic increase in E. faecium in the current study. This extreme microbiological shift in the historical 10:1 ratio of E. faecalis and E. faecium is in concordance with another study from north India [7] but in contrast with studies reported from other parts of India [17,18].
The incidence of VRE is 2.7% in the current study which was not present in the previous published study. The mean age for VRE patients was 10 years more than non-VRE patients but the difference was not statistically significant (p-value>0.05). Still, this is in contrast to other long duration study in which mean age for VRE patients was 62 years. In our study there was no significant correlation with sex of the patients but other studies have found female sex as predisposing factor for VRE [18]. All the enterococcal isolates were obtained from inpatients as majority of the samples (99%) were from indoor patients only. This could be attributed to increased emergence of E. faecium in the present study. Prolonged hospitalisation and admission in intensive care unit were identified as significant (p-value<0.05) risk factors for VRE bacteriuria which have been identified in other studies as well [7,19].
Incidence of high level aminoglycoside resistance was detected in 81.4% (57) of the isolates. The rate is higher than the data published in previous study but in concordance with other studies [14,17]. E. faecalis isolates were observed to be more resistant than E. faecium as reported by others [19].
The antimicrobial susceptibility rate against nitrofurantoin was 81.4% which is in concordance with other studies [15,20,21]. It showed better susceptibility pattern in comparison to high dose of gentamycin, ciprofloxacin, teicoplanin, penicillin and erythromycin for both the species and the result was in concordance with other study results [22,23]. Though, the prevalence of VRE has increased over the years but susceptibility of NF has remained the same against Enterococcus spp. A total of 21 out of 26 (80.7%) among the VRE and eight out of 40 (18%) among the Vancomycin Susceptible Enterococcus (VSE) were susceptible to NF [Table/Fig-3].
The stupendous activity of linezolid and nitrofurantoin against VRE has been previously reported also [20,21]. Moreover, there was no difference in susceptibility pattern among the two common species of enterococci i.e. E. faecalis and E. faecium which is in concordance with other studies [17,18].
The advantage of NF is its action at multiple sites and multiple levels of the organism. This includes inhibition of bacterial enzymes involved in carbohydrate synthesis. In higher concentration, it inhibits nucleic acid and total protein synthesis by the nonspecific attack on bacterial ribosomal proteins [12]. Hence, clinical drug resistance emerges slowly. There is no cross resistance between nitrofurantoin and other antimicrobial agents also. Side effects such as anorexia, nausea and vomiting occur at rates <0.001% especially with macrocrystal formulations [24]. It is contraindicated in patients with renal failure with creatinine clearance rate of 60 ml/min. However, recent studies indicate that nitrofurantoin can be used among patients with creatinine clearance as low as 40 ml/min [25].
Our results indicate that susceptibility to penicillin, ciprofloxacin, aminoglycoside, teicoplanin, erythromycin has dropped to less than 20% for all enterococcal isolates. So, NF may be a reliable option for uncomplicated acute cystitis, which is in agreement with recommendations by other authors [26,27]. The complete susceptibility to linezolid is elicited in our study supports the recommendations of NF as a reserved drug to treat VRE in complicated cases of UTI such as pyelonephritis, urinary tract infection with bacteremia, or urosepsis etc., [21,24,28]. However, one must differentiate between colonisation and infection before the start of treatment.
Limitation
The current study included all positive urine cultures irrespective of symptomatic or asymptomatic bacteriuria. Catheter Associated UTI (CAUTI) could not be established due to lack of data.
Conclusion
Nitrofurantoin is effective even after 50 years with minimal resistance. It could serve as a useful therapeutic agent against rapidly emerging VRE especially in uncomplicated UTI. Linezolid could be used as reserve drug for complicated enterococcal UTI to prevent emergence of resistance.