Cytological Diagnosis of an Uncommon High Grade Malignant Thyroid Tumour: A Case Report
Ruchi Nagpal1, Manju Kaushal2, Sawan Kumar3
1 Senior Resident, Department of Pathology, PGIMER, Dr RML Hospital, New Delhi, India.
2 Professor, Department of Pathology, PGIMER, Dr RML Hospital, New Delhi, India.
3 Senior Resident, Department of Pathology, PGIMER, Dr RML Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ruchi Nagpal, H.No-118, Sector-13, Kurukshetra-136118, Haryana, India. E-mail : ruchi2001@gmail.com
Anaplastic Thyroid Carcinoma (ATC) is a relatively uncommon highly malignant tumour originating from the follicular cells of thyroid gland having poor prognosis. It accounts for 2% to 5% of all thyroid carcinomas and patients typically present with a rapidly growing anterior neck mass with aggressive symptoms. A 53-year-old male presented with diffuse neck swelling measuring 8x6 cm and right cervical lymph node measuring 2x2 cm since one month which was associated with dyspepsia and dyspnoea. Ultrasound and Contrast Enhanced Computed Tomography (CECT) neck revealed enlarged right lobe of thyroid and multiple enlarged cervical lymph nodes with soft tissue density nodules in bilateral lungs. Fine Needle Aspiration (FNA) from the swelling revealed giant cell, spindle cell and squamoid pattern. Focal areas showed follicular epithelial cells arranged in repeated microfollicular pattern suggesting an underlying follicular neoplasm. FNAC smears from the lymph node also revealed similar findings. Based on the cytomorphological and radiological findings, final diagnosis of ATC probably arising from underlying follicular carcinoma with cervical lymph node and lung metastasis was given. FNAC leads to prompt and definitive diagnosis, so that therapy can be initiated as soon as possible for better outcome. Multimodality therapy (surgery, external beam radiation, and chemotherapy) is the mainstay of treatment.
Anaplastic thyroid carcinoma, Fine needle aspiration cytology, Follicular neoplasm
Case Report
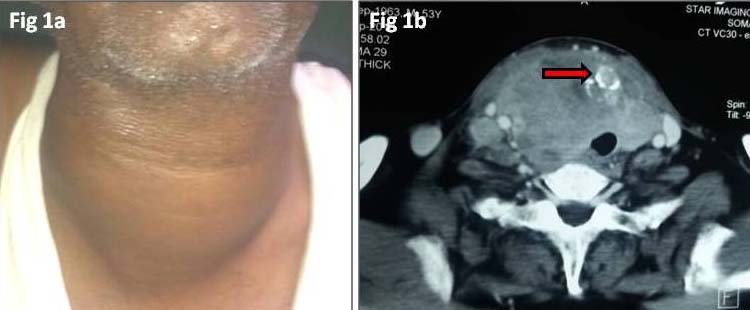
A 53-year-old male patient presented with a diffuse neck swelling since one month, associated with dyspepsia and dyspnoea. On examination the swelling was fixed, had diffuse margins, was hard in consistency and measured 8x6 cm [Table/Fig-1a]. A right upper cervical lymph node enlargement was also noted which was mobile, firm in consistency, non tender and measured 2x2 cm. Thyroid function tests revealed high serum free T4 (2.06 ng/dl) and low serum TSH (0.016 mU/ml). Serum calcitonin was <2.0 pg/ml. Ultrasound neck showed diffuse enlargement of predominantly right lobe of thyroid which was replaced by hyperechoic mass lesion measuring 5.5 x 5.3x5.1 cm with few partially calcified nodules with extension into superior mediastinum. Contrast Enhanced CT neck showed large heterogeneously enhancing mass lesion measuring 10.6x6.6x8.3 cm arising from right lobe, isthmus and portion of left lobe [Table/Fig-1b]. Multiple enlarged right cervical lymph nodes were also noted at level III and IV, largest measuring 15x30 mm, having features similar to primary lesion. Multiple soft tissue density nodules were seen in random distribution in bilateral lungs.
a) Anterior neck swelling 8X6 cm; b) CECT neck showing heterogeneously enhancing mass lesion in front of cervical vertebra measuring 10.6X6.6X8.3 cm arising from right lobe, isthmus and portion of left lobe (arrow showing foci of calcification).
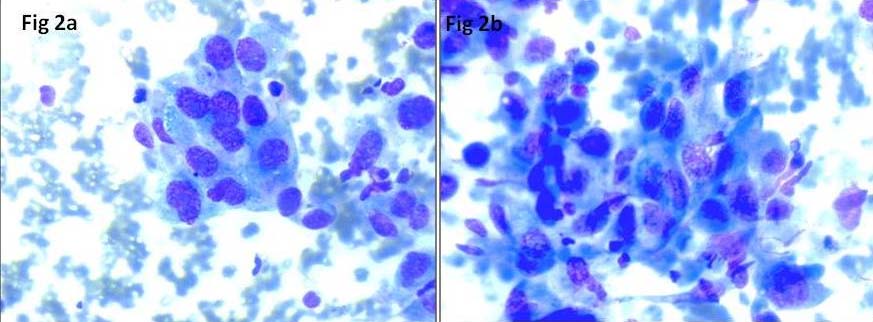
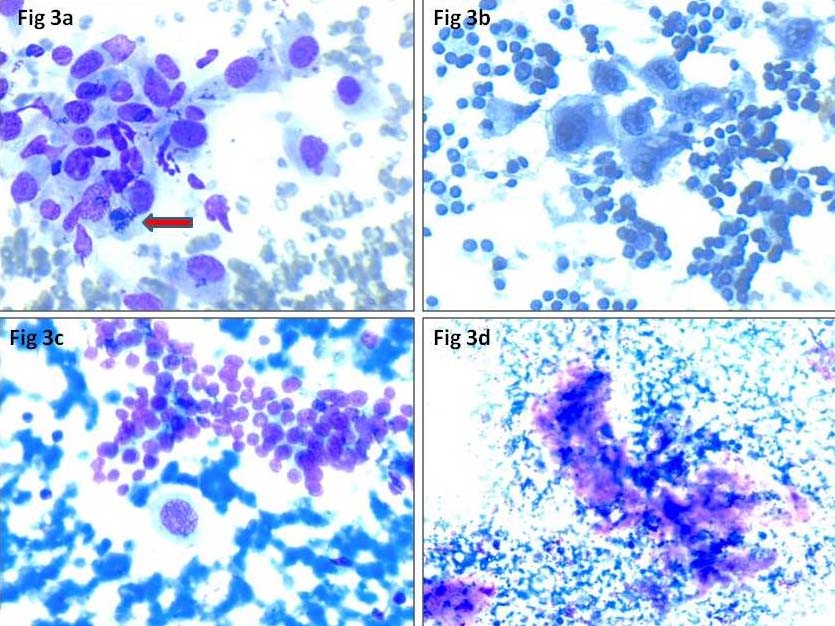
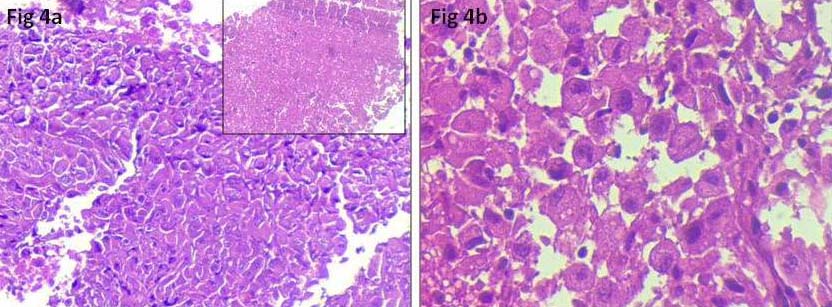
FNA smears performed from the thyroid swelling and cervical lymph node, were cellular comprising of highly pleomorphic follicular epithelial cells arranged in large tissue fragments, clusters and singly scattered. Cells were large, having round to oval nuclei, coarse chromatin, prominent nucleoli and moderate to abundant cytoplasm [Table/Fig-2a]. Few bizarre cells, multinucleated giant cells, spindle cells [Table/Fig-2b], squamoid cells and mitotic figures [Table/Fig-3a] were seen. Occasional foci showing repeated microfollicular pattern of follicular epithelial cells were also noted [Table/Fig-3b]. Few clusters of benign follicular epithelial cells [Table/Fig-3c] were seen in the background of blood mixed with chunks of thick colloid, necrosis [Table/Fig-3d] and inflammatory cells. A cell block was prepared from the thyroid swelling which revealed highly pleomorphic cells with round to oval nuclei, coarse chromatin, prominent nucleoli and moderate amount of cytoplasm with areas of necrosis [Table/Fig-4a,b]. Final diagnosis of ATC (possibly dedifferentiation of follicular carcinoma) with right cervical lymph node metastasis was made. Patient was started on doxorubicin and radiotherapy. Surgery was not performed due to ill defined plane of resection and lung metastasis. Follow up details were not available as the patient left without intimation.
a) Anaplastic carcinoma thyroid with marked pleomorphic malignant epithelial cells with prominent nucleoli. (MGG stain, 40X); b) Few spindle cells interspersed in between malignant cells (MGG stain, 40X).
a) Arrow showing atypical mitotic figure (MGG stain, 40X); b) Occasional foci showing repeated microfollicular pattern of follicular epithelial cells (Pap stain, 40X); c) Cluster of benign follicular epithelial cells (MGG stain, 40X); d): Focal area of necrosis (MGG, 20X).
a): Cell block showing highly pleomorphic cells, inset showing area of necrosis (H&E stain, 10X); b) High power showing highly pleomorphic cells with round to oval nuclei, coarse chromatin, prominent nucleoli and moderate amount of cytoplasm (H&E, 40X).
Discussion
ATC is also known as undifferentiated, dedifferentiated or sarcomatoid carcinoma. ATC is an uncommon, aggressive tumour which constitutes 2 to 5% of all thyroid tumours but is invariably fatal [1,2]. It may occur from dedifferentiation of existing well differentiated carcinoma, but such transformation is not frequently seen and is reported in <2% of cases [3]. Demeter JG et al., reported previous or concurrent thyroid disorders in 76% of ATCs and association with Well Differentiated Thyroid Carcinoma (WDTC) in 46% of cases [4]. All the subtypes of thyroid carcinoma can progress to ATC but papillary and follicular carcinoma are the most common types to undergo this transformation [3]. The incidence of ATC has decreased in recent times. Improved socioeconomic conditions and iodine prophylaxis is suggested as the main reason for the decline as ATC is more common in iodine deficient regions. Few authors postulated the increased rate of thyroid resection for other conditions as one of the reason as it eliminates the transformation of WDTC to ATC. New diagnostic techniques which distinguish the previously described cases of ATC from lymphoma and medullary thyroid carcinoma is also described [5,6].
ATC is seen in elderly with peak incidence in 6th to 7th decade and is more common in females (55-77%) [7]. Patients usually present with rapidly growing neck mass with extra thyroid extension leading to hoarseness of voice, dysphagia and dyspnoea [5,7]. Pathogenesis of ATC is not completely understood [8]. Somatic mutations in the promoter of Telomerase Reverse Transcriptase (TERT), mutation in CTNNB1 (catenin) gene leading to active Wnt signal, inactivation of tumour suppression genes p53 and PTEN, gain of function mutations in Phosphatidylinositol-4,5 Bisphosphate 3 Kinase Catalytic Subunit Alpha (PIK3CA) have been described [2].
FNAC provides an early diagnosis and sensitivity of FNAC for thyroid malignancy ranges from 61% to 97.7% [7,9,10]. On FNAC three major patterns are recognised: giant cell, spindle cell and squamoid pattern. Majority of the cases show admixture of these patterns. However, the cell type doesn’t affect the treatment and prognosis of the patient [2]. Regardless of the type, cells have highly pleomorphic nuclei, have coarsely granular clumped chromatin, prominent nucleoli and occasional intranuclear inclusions. Multinucleation, multilobation and numerous mitotic figures are common. Carcinoma with spindle cell pattern and abundant collagenous stroma tend to be paucicellular or acellular, causing difficulty in diagnosis. Few authors have suggested that all ATC contain foci of WDTC but the inadequate sampling of the specimen leads to inability to diagnose [11-13]. Papillary thyroid carcinoma is considered to be the most common type of thyroid cancer associated with ATC; tall cell variant being more common [14]. Variable immunophenotype is seen in ATC. Around 40 to 100% of cases show immunoreactivity to cytokeratin. Spindle cell component express vimentin whereas squamoid cells express Epithelial Membrane Antigen (EMA) and Carcinoembryonic Antigen (CEA). Interspersed non neoplastic thyroid follicular epithelial cells may give false positivity to thyroglobulin. ATC cells are not reactive for TTF 1, calcitonin, thyroglobulin and RET/PTC oncoprotein. PAX8 (also known as paired box gene 8) has a useful role and is positive in 79% of ATCs and in up to 92% of ATCs showing squamoid features [2]. Our patient was an elderly male and presented with rapidly progressive neck mass with compression symptoms and metastasis to cervical lymph node and bilateral lungs. Prompt diagnosis was offered by FNAC. Patient was started on chemo-radiotherapy. As ATCs are rapidly progressive tumours, multimodality treatment comprising of surgery and when possible combined with radiation and chemotherapy is generally given. Direct invasion to surrounding tissues is found in 70% of patients. Systemic metastasis occur in 75% cases, lung being the most common site [6,7]. Overall prognosis remains poor despite multimodal therapy.
Conclusion
Anaplastic carcinoma of thyroid is an uncommon but markedly aggressive tumour. Transformation from pre-existing carcinoma is rare and reported in <2% of cases. FNAC plays a pivotal role in making early and accurate diagnosis facilitating early treatment.
[1]. Chiacchio S, Lorenzoni A, Boni G, Rubello D, Elisei R, Mariani G, Anaplastic thyroid cancer: Prevalence, diagnosis and treatmentMinerva Endocrinol 2008 33:341-57. [Google Scholar]
[2]. Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S, Update on anaplastic thyroid carcinoma: Morphological, molecular, and genetic features of the most aggressive thyroid cancerInt J Endocrinol 2014 2014:790-834. [Google Scholar]
[3]. Saunders CA, Nayar R, Anaplastic spindle-cell squamous carcinoma arising in association with tall-cell papillary cancer of the thyroid: A potential pitfallDiagn Cytopathol 1999 21:413-18.Erratum in: Diagn Cytopathol 2000; 22 136 [Google Scholar]
[4]. Demeter JG, De Jong SA, Lawrence AM, Paloyan E, Anaplastic thyroid carcinoma: Risk factors and outcomeSurgery 1991 110(6):956-63. [Google Scholar]
[5]. Pitt SC, Moley JF, Medullary, anaplastic, and metastatic cancers of the thyroidSemin Oncol 2010 37(6):567-79. [Google Scholar]
[6]. Patel KN, Shaha AR, Poorly differentiated and anaplastic thyroid cancerCancer Control 2006 13(2):119-28. [Google Scholar]
[7]. Piromchai P, Ratanaanekchai T, Kasemsiri P, Diagnosis and Treatment of Anaplastic Thyroid CarcinomaInternational Journal of Clinical Medicine 2012 3:69-73. [Google Scholar]
[8]. Mobeen Alwani, Gunvanti B, Rathod Diagnosis of anaplastic thyroid carcinoma on fine needle aspiration cytology – A rare case reportIAIM 2015 2(3):183-18. [Google Scholar]
[9]. Feng G, Laskin WB, Chou PM, Lin X, Anaplastic thyroid carcinoma with rhabdoid featuresDiagn Cytopathol 2015 43(5):416-20. [Google Scholar]
[10]. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancerThyroid 2012 22(11):1104-39. [Google Scholar]
[11]. Carcangiu ML, Steeper T, Zampi G, Rosai J, Anaplastic thyroid carcinoma. A study of 70 casesAm J Clin Pathol 1985 83(2):135-58. [Google Scholar]
[12]. Nishiyama RH, Dunn EL, Thompson NW, Anaplastic spindle-cell and giant-cell tumours of the thyroid glandCancer 1972 30(1):113-27. [Google Scholar]
[13]. Ibanez ML, Russell WO, Albores-Saavedra J, Lampertico P, White EC, Clark RL, Thyroid carcinoma—biologic behavior and mortality. Postmortem findings in 42 cases, including 27 in which the disease was fatalCancer 1966 19(8):1039-52. [Google Scholar]
[14]. Moreno Egea A, Rodriguez Gonzalez JM, Sola Perez J, Soria Cogollos T, Parrilla Paricio P, Prognostic value of the tall cell variety of papillary cancer of the thyroidEur J Surg Oncol 1993 19(6):517-21. [Google Scholar]