Clear Cell Adenocarcinoma of Female Urethra
Sharada Raju Rane1, Ashwini Nivrutti Ghodke2, Sharvari Vishwasrao3
1 Associate Professor, Department of Pathology, BJGMC, Pune, Maharashtra, India.
2 Assistant Professor, Department of Pathology, BJGMC, Pune, Maharashtra, India.
3 Postgraduate Resident Doctor, Department of Pathology, BJGMC, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ashwini Nivrutti Ghodke, Assistant Professor, Department of Pathology, BJGMC, Pune-411001, Maharashtra, India.
E-mail: dr.ashwinighodke@gmail.com
Primary malignancies of female urethra are infrequent, constituting a fraction of less than 1% of genitourinary malignancies. Primary clear-cell adenocarcinoma of the urethra, is even rarer, that histomorphologically resembles clear-cell carcinoma of the female genital tract, occurs predominantly in women and is associated with a relatively poor prognosis. The histogenesis of this rare urethral neoplasm has not been completely determined. Various hypotheses concerning the origin have been postulated, including (1) diverticular origin (2) mullerian origin (3), glandular differentiation of urothelium or urothelial carcinoma. Here, we report a case of 67-year-old female with obstructive urinary symptoms and pain in abdomen, diagnosed with adenocarcinoma of urethra. Immunohistochemistry (IHC) workup of the tumour was done to find the origin of the tumour.
Immunohistochemistry, Mullerian rests, Urethral Carcinoma, Urogenital Tract
Case Report
A 67-year-old woman presented with obstructive urinary symptoms and pain in abdomen. No hematuria was noted. CT scan and USG pelvis and abdomen showed peripheral enhancing lesion at bladder neck and along urethra suggestive of infective/inflammatory aetiology. Heterogeneously enhancing nodular thickness was seen along anterior bladder wall along with abdominal and inguinal lymphadenopathy. Vesicourethroscopy examination showed tight urethra; complete periurethral induration with mass involving trigone of bladder, diverticula was also noted. Biopsy of tumour was done and a diagnosis of adenocarcinoma was made.
Following this, the patient received two chemotherapy cycles. CT abdomen after 2nd chemotherapy showed residual urinary bladder wall thickening with invasion of vagina and vesico vaginal fistula. Patient underwent bladder exenteration surgery with hysterectomy and bilateral salpingo-oophorectomy with ureteric implantation into rectum. Postoperative period was uneventful.
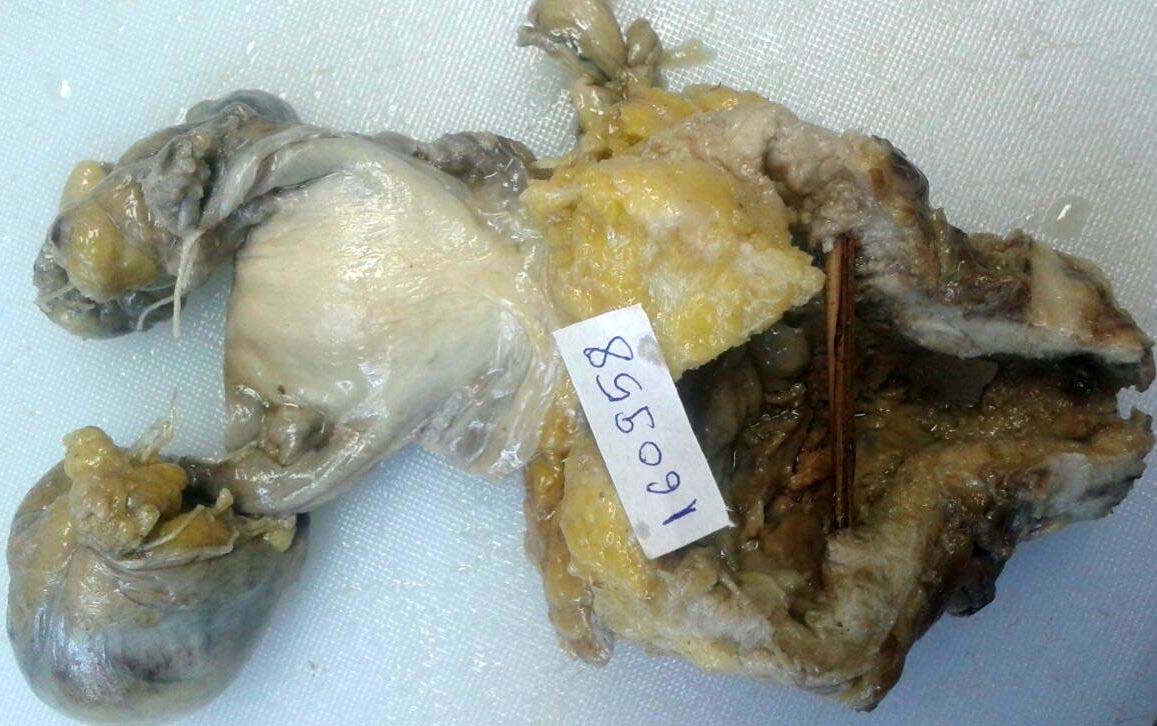
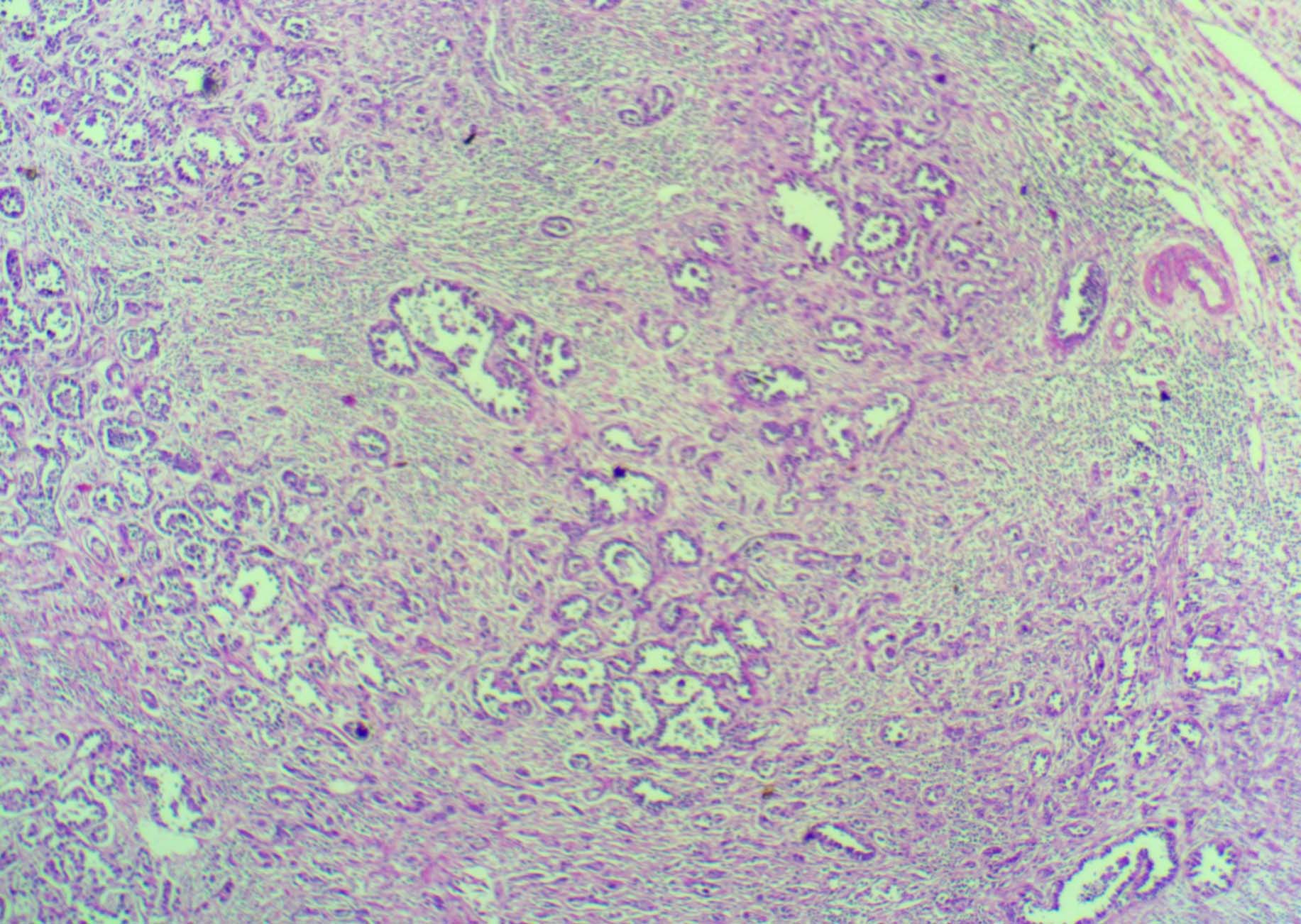
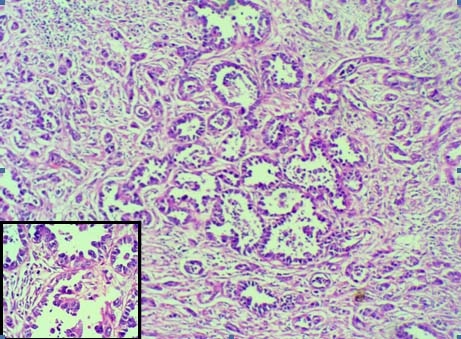
Gross examination of the specimen revealed ulcerative growth in urethra, measuring 5 x 4 x 3 cm, grey white in colour and extending into the lower part of bladder. A perforation of 3 cm in diameter was noted in the anterior vaginal wall [Table/Fig-1]. Final histopathology reports revealed areas with cells in diffuse arrangement with round to oval hyperchromatic, pleomorphic nuclei with eosinophilic cytoplasm [Table/Fig-2,3]. Few cells with clear cytoplasm and Hob nail pattern were also noted [Table/Fig-3].
Gross–ulcerative growth in urethra (arrow).
Scanner view of urethral clear cell adenocarcinoma (H&E 4X).
Urethral clear cell adenocarcinoma (H&E 10X); Inset –Hob nail pattern (H&E 40X).
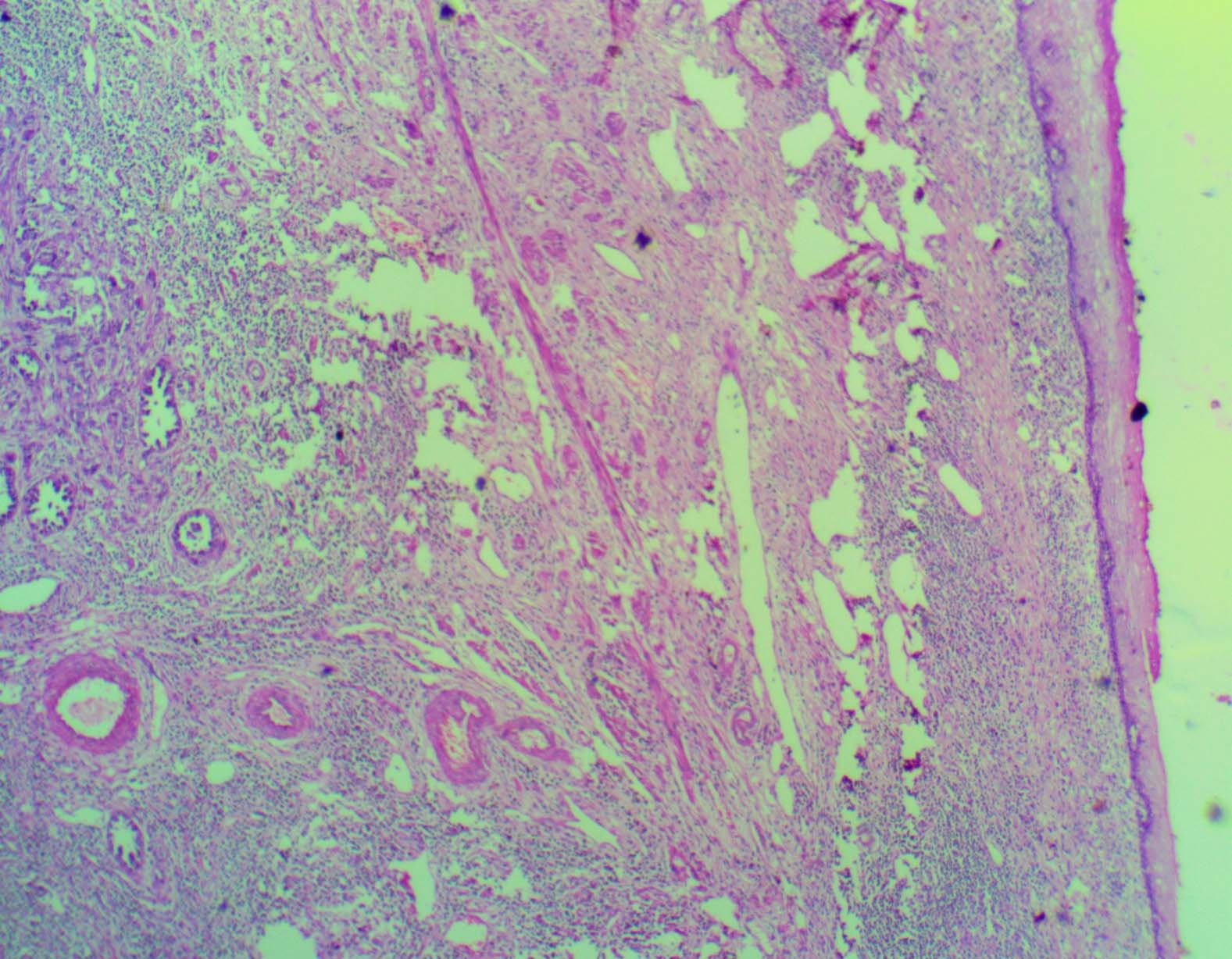
On microscopic assessment of section from perforation in anterior vaginal wall, it showed clear cell adenocarcinoma extending into vaginal vault. But the vaginal epithelium was free of tumour [Table/Fig-4]. Bladder sections revealed severe follicular cystitis. Bilateral ureteric margins were free of tumour. No lymphovascular emboli were noted.
Overlying free vaginal epithelium with underlying adenocarcinoma (H&E 4X).
IHC study done to find out origin of the tumour revealed: CK7 – Positive [Table/Fig-5], CK20 – Negative, PSA – Negative, CDX2 – Negative. Follow up of the patient was uneventful.
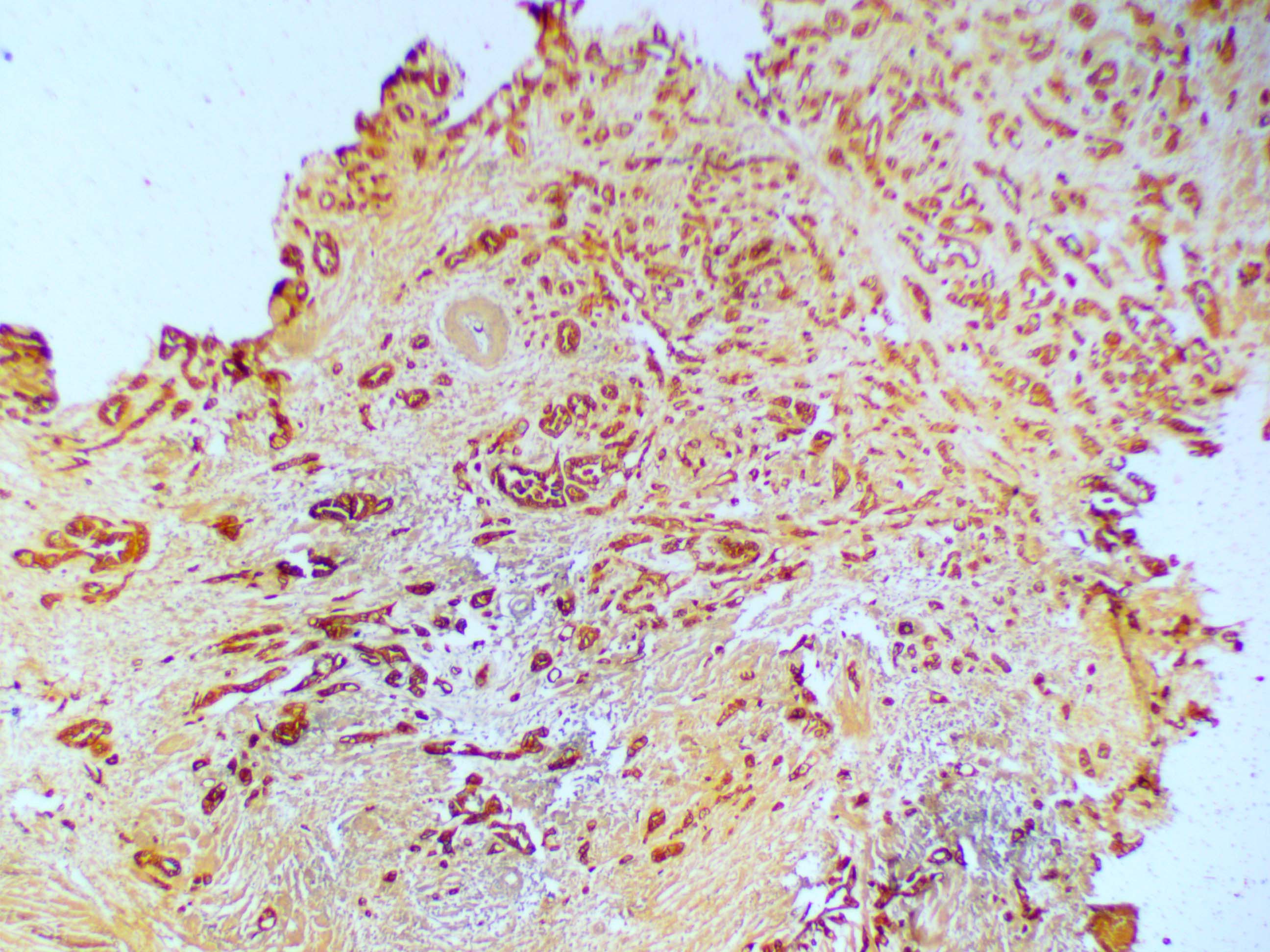
Discussion
Primary urethral cancer is a rare disease and occurs more frequently in females with M: F=1:4 [1]. In vast majority of primary urethral cancer, squamous cell carcinoma is the most common histologic type in either sex or any race [2]. Clear cell adenocarcinoma accounts for 0.003% of malignant tumours occurring in female urogenital tract, with the average age of occurrence in females being 58 years [3,4].
Adenocarcinoma is the most predominant histological type of urethral cancer arising from a diverticula of the urethra, whereas other types such as squamous cell carcinoma and transitional cell carcinoma being less common [1,4]. According to Clayton M et al., and Evans KJ et al., 46-56% of the urethral diverticulum associated carcinomas are adenocarcinoma and only 15-18% is squamous cell carcinoma [5,6].
Adenocarcinoma can be further divided into columnar/mucinous and clear cell types [7]. Clear cell type adenocarcinoma accounts for only 10% of urethral cancer and has a clear association with diverticulum and is the most common malignancy arising from them [1,4]. Incidence of urethral diverticulum is 1-6% in the total female population [1]. The histogenesis of primary urethral clear cell adenocarcinoma has not been conclusively determined [8]. Various theories on its origin from urethral diverticula of the paraurethral ducts or glands (Skene’s gland) [7] or from mullerian rest or urothelial metaplasia have been suggested [8].
Primary urethral carcinoma arising from a diverticulum being even uncommon with only 100 reported cases since 1951 [1]. In our case, tumour were negative for PSA, CK20, CDX2, Positive for CK7 and a diverticula was reported on cystourethrographic examination. The present findings support the theory that the female clear cell adenocarcinoma arises from diverticula.
Most information has been gained from single case reports and small case series. In 1985, Young and Scully first introduced the term clear cell adenocarcinoma because of their similar appearance to that of female genital tract of mullerian origin [8]. The development of clear cell adenocarcinoma of urethra has not been conclusively determined [8]. Many theories of its origin from paraurethral ducts/glands (Skene’s gland) [7] or mullerian rest/urothelial metsplasia [8] have been suggested. Paraurethral ducts/glands (Skene’s glands) are considered embryologically homologous to the male prostate glands, because some of the normal ducts stain positively for PSA [7]. Based on these immunohistological findings and distinct subtypes, different authors suggested that a distinct subsets of paraurethral ducts exists some of which are homologous to male prostate [7] and female adenocarcinoma has more than one tissue of origin with minority arising from Skene’s gland [9]. Incidence of urethral diverticulum is 1 -6% in the total female population [1].
Conclusion
Urethral cancer is very rare and its diagnosis can be delayed because of vague and nonspecific symptoms. Urethral carcinoma is prone to recur and distant metastasis so, its diagnosis, staging and treatment is highly essential as advanced cases have very bad prognosis. Hence, we report this case to focus on the importance of histopathology for its diagnosis and increase its awareness.
[1]. Weng WC, Wang CC, Ho CH, Chang HC, Chen SC, Yu HJ, Clear cell carcinoma of female urethral diverticulumJournal of Formosan Medical Association 2013 112:489-91. [Google Scholar]
[2]. Scantling D, Ross C, Jaffe J, Primary Clear Cell Adenocarcinoma of a Urethral Diverticulum Treated with Multidisciplinary Robotic Anterior Pelvic ExenterationCase Reports in Medicine 2013 :387591 [Google Scholar]
[3]. Satyanarayan A, Redd L, Dyer A, Wright A, Walker J, Adenocarcinoma of the urethra with mucinous featuresReviews in Urology 2015 17(1):38-41. [Google Scholar]
[4]. Venyo AK-G, Clear cell adenocarcinoma of the urethra: review of the literatureInternational Journal of Surgical Oncology 2015 2015:790235 [Google Scholar]
[5]. Clayton M, Siami P, Guinan P, Urethral diverticular carcinomaCancer 1992 70:665-70. [Google Scholar]
[6]. Evans KJ, McCarthy MP, Sands JP, Adenocarcinoma of a female urethral diverticulum: case report and review of the literatureJ Urol 1981 126:124-26. [Google Scholar]
[7]. Kato H, Ogihara S, Kobayashi Y, Toguri AG, Igawa Y, Nishizawa O, Carcinoembryonic antigen positive adenocarcinoma of a female urethral diverticulum: case report and review of the literatureInt J Urol 1998 5(3):291-93. [Google Scholar]
[8]. Young R, H and Scully R. E. “Clear cell adenocarcinoma of the bladder and urethra. A report of three cases and review of the literature,”American Journal of Surgical Pathology 1985 9(11):816-26. [Google Scholar]
[9]. Kato H, Kobayashi S, Islam AM, Nishizawa O, Female para-urethral adenocarcinoma: Histological and immunohistochemical studyInternational Journal of Urology 2005 12:117-19. [Google Scholar]