Inflammation of the appendix is a common cause of abdominal pain especially in adolescents and young adults that often requires surgical treatment. There is an approximately 6-7% lifetime risk of appendicitis to the population globally [1,2]. Most of the observations available on appendix till date in human beings and animals were focused on histopathology and immunohistochemistry. In the previous histopathological observations, authors reported significant ultrastructural damage of neurons and interstitial cell of Cajal networks in appendicitis [3,4]. These observations point toward alteration in motility/contractility in appendicitis. In our earlier observations, we reported mechanisms involved in contractility of longitudinal muscle strips of normal human vermiform appendix involving muscarinic and serotonergic mechanisms [5]. Beside, structural changes in the muscle of appendix, variety of inflammatory agents like Prostaglandins (PGs), histamine, kinins etc., are expressed that may affect the contractility in the inflamed appendix [6].
But till date, there are no reports available that demonstrate the mechanisms mediating the contractility of appendix in inflamed state. Therefore, the present in vitro study was undertaken to investigate the underlying mechanisms involved in mediating the contractility of longitudinal muscle strips of inflamed vermiform appendix of human beings, using ACh, 5-HT and histamine as chemical transmitters.
Materials and Methods
The present in vitro experimental study on human vermiform appendix was conducted in the period between May 2013 to October 2014. This study was performed as per the guidelines framed by the Ethical Clearance Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Criteria for Selection of Inflamed Vermiform Appendix Samples
Inclusion criteria: Human appendix specimens obtained by means of laparotomy from clinically diagnosed cases of acute/ chronic appendicitis patients of age between 15-50 years of both sexes were considered initially. But, only those specimens of appendices were included in the study which was diagnosed as appendicitis on microscopy.
Exclusion criteria: Patients of younger age (<15 years) and older age (>50 years) were excluded. Clinically diagnosed cases of acute/chronic appendicitis but histopathologically normal were excluded from the present study. Gangrenous, perforated, tumourous appendices were excluded from the study by naked eye examination done by operating surgeon in the operation theatre. Specimen resected by means of laparoscopic surgery was also excluded.
Collection and Transportation of Appendix Samples
A proper informed consent was taken from patients in all the cases of appendicitis on a bilingual (English/Hindi) consent form. In the current study, 24 samples of human inflamed vermiform appendix were recruited as per the inclusion criteria. Appendix samples were collected from operation theatre of Department of General Surgery of Sir Sunder Lal Hospital, Banaras Hindu University, Varanasi immediately after appendicectomy. Appendices were cut in two halves, proximal half and distal half in the operation theater. The proximal half of the appendix sample was kept in a wide mouthed bottle containing ice-cold Krebs-Ringer solution pre-bubbled with 100% O2. Thereafter, the bottle with sample was carried to the Department of Physiology, Institute of Medical Sciences, Banaras Hindu University for contractility study. The distal half of the appendix sample was transported to Department of Pathology, Institute of Medical Sciences, Banaras Hindu University for confirmation of diagnosis of appendicitis by microscopy.
Preparation of the Longitudinal Muscle Strips from Inflamed Appendix
The appendicular tissue was cleaned and three longitudinal muscle strips (10-15×2-3 mm each) were prepared from each appendix. These three strips were used to test three different agonist (ACh, 5-HT and histamine).
Recording of Appendicular Muscle Contractions
The methodology used to record the muscle contractions was followed as per previous reports [7]. In brief, one end of the muscle strip was tied to a glass tube via a thread and a piece of thread was also tied to other end of the muscle strip. Subsequently, the tube with the longitudinal muscle strips of inflamed appendix was placed in Dale’s organ bath (50 ml) containing Krebs-Ringer solution which was continuously bubbled by 100% O2. The temperature of the solution was maintained at 37±0.5°C. Then, the other end of muscle strip was tied to a Statham’s force displacement transducer which was electrically connected to physiograph (Bio-devices, Dellsoft Technologies Pvt. Ltd., India). The tissue was allowed to stabilise for 30 minutes under an initial tension of 0.5 gram and control contraction was recorded on a moving paper on physiograph.
Experimental Protocol
In the present study, experiments were divided into three groups, one for each agonist (ACh, 5-HT and histamine). Then, each group was subdivided into two subgroups (subgroup 1 and subgroup 2). In subgroup 1 of each group, dose-response experiments of agonist were performed to determine dose of agonist that elicited maximum amplitude of contraction. In subgroup 2 of each group, the agonist induced muscle contractions were obtained before and after pretreatment with appropriate antagonist (atropine, ondansetron and chlorpheniramine maleate).
In Group 1, subgroup 1 set of experiments, after initial stabilisation of the muscle strip for 30 minutes, different doses of ACh in increasing order (0.1, 1, 10, 100 µM) were used and muscle contractions were recorded for 5 minutes at each dose (n=6). In between two doses, the muscle strip was washed twice at an interval of 10 minutes with Krebs-Ringer solution and allowed to stabilise for another 10 minutes before recording muscle contractions with next higher dose of ACh.
In Group 1, subgroup 2 set of experiments, after initial stabilisation of the muscle strip for 30 minutes, ACh (10 µM) induced muscle contractions were recorded for 5 minutes. Subsequently, the muscle strip was washed twice at an interval of 10 minutes with Krebs-Ringer solution and stabilised for another 10 minutes before it was exposed to atropine (100 µM) for 5 minutes. Thereafter, ACh (10 µM) induced contractile response was obtained again and values were compared with the values recorded before.
In subgroup 1 set of experiments of Group 2 and 3, the dose response of 5-HT (0.1, 1, 3 µM) and histamine (0.01, 0.1, 1, 10 µM) were obtained using protocols similar to ACh (as in Group 1, subgroup 1) respectively.
In subgroup 2 set of experiments of Group 2, 5-HT (1 µM) induced muscle contractions were recorded before and after exposure to ondansetron (10 µM) and values were compared. Further, in subgroup 2 set of experiments of Group 3, histamine (10 µM) induced muscle contractions were recorded before and after exposure to chlorpheniramine maleate (100 µM) and were compared. Protocols followed for subgroup 2 set of experiments of Group 2 and 3 were similar to experimental protocol of subgroup 2 set of experiments of Group 1.
Further, at the end of each experiment, the muscle strip was detached from the glass tube and Statham’s force transducer. Then, the muscle strip was soaked on the blotting paper for 10 seconds. Its weight was obtained on an electronic weighing machine. The muscle contractions were quantified and expressed as gram/gram (g/g) of muscle tissue.
Drugs and Solutions
Acetylcholine chloride (ACh), Atropine Sulphate (Atropine), Serotonin Creatinine Sulphate Monohydrate (5-HT) and Histamine Dihydrochloride (Histamine) were procured from Sigma chemicals Co., St. Louis, MO, USA. Ondansetron Hydrochloride (Ondansetron) was bought from Cipla Limited, Mumbai, India and Chlorpheniramine Maleate (CPM) was obtained from Alkem Laboratories Ltd., Mumbai, India. Krebs-Ringer solution was prepared using following chemicals in appropriate concentration in milimole/liter (mM/l): Sodium Chloride (NaCl), 119; Potassium Chloride (KCl), 4.7; Calcium Chloride Dihydrate (CaCl2.2H2O), 2.5; Potassium Dihydrogen Phosphate (KH2PO4), 1.2; Magnesium Sulfate Heptahydrate (MgSO4.7H2O), 1.2; Sodium Bicarbonate (NaHCO3), 5; Glucose, 11. The stock solutions of 10 mM concentration of each agonists and antagonists were prepared in distilled water and were kept in refrigerator. During experiment, stock solutions were diluted to appropriate concentration with Krebs-Ringer solution. Analytical grade chemicals were used in the present study.
Statistical Analysis
In the present study, data were expressed as Mean±Standard Error of Mean (SEM). GraphPad Prism 6 was used for statistical analysis. Data analysis of the dose-response was performed using One-Way Analysis Of Variance (ANOVA) and Students t-test for paired observations was used to compare between two groups (agonist induced contractions before and after exposure to antagonist). When the value of p was < 0.05 in a statistical test, it was considered significant.
Results
1. Ach Produced Muscle Contractions in Inflamed Vermiform Appendix
Contractions elicited in the longitudinal muscle strips of inflamed vermiform appendix by ACh (0.1-100 µM) demonstrated dose-dependent increase in amplitude upto 10 µM. Thereafter, increase in the dose of ACh up to 100 µM (n=6 at each dose) caused fall in amplitude of muscle contractions. The maximum contraction was observed at 10 µM of ACh. The amplitude of contractions obtained at each dose of ACh were significantly different (p<0.05, [Table/Fig-1a]).
a) shows dose-responses of ACh (0.1-100 µM) elicited muscle contractions (g/g) in inflamed vermiform appendix. The values of amplitude of contractions are represented as Mean ± SEM (n=6) at each dose. The contractions at each dose are significantly different (p<0.05, One-way ANOVA). An asterisk (*) indicates significant difference among the responses obtained at each dose; b) demonstrates the original recording of an experiment of ACh (10 µM) induced contractions before and after exposure to atropine (100 µM). Scale for measuring the tension and time are shown at the top left corner; c) demonstrates Mean±SEM value (n=6) of the ACh (10µM) elicited contractions (g/g) before and after exposure to atropine (100 µM). Muscle contractions produced by ACh in longitudinal muscle strips of inflamed appendix were significantly blocked by atropine pre-treatment (p<0.05, Student t-test for paired observations). Asterisk (*) indicates significant difference from before values.
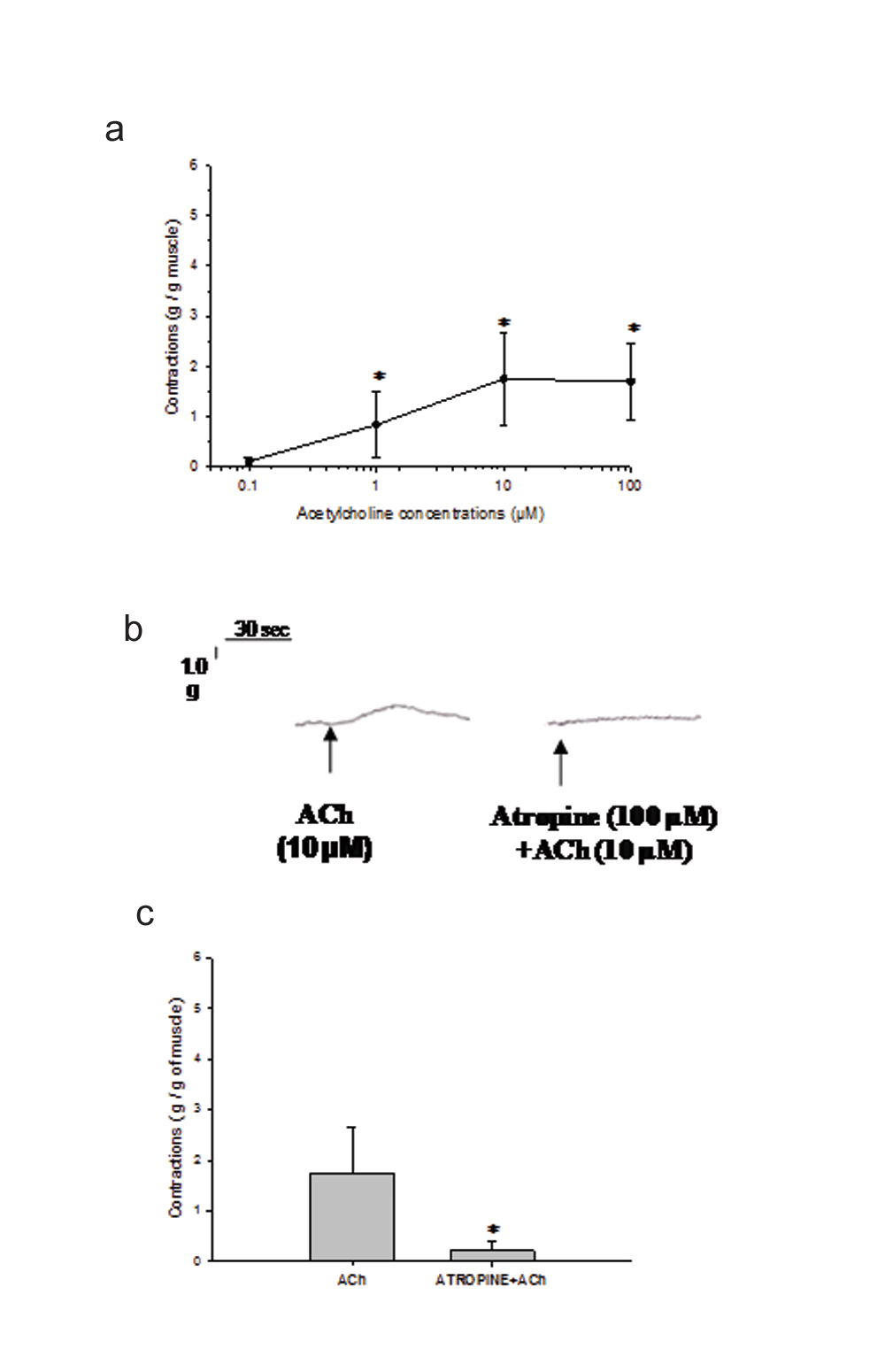
2. Ach Induced Contractions were Blocked by Atropine
In this group of experiments also, the amplitude of contractions elicited by ACh (10 µM) were almost similar to those observed in dose-response experiments [Table/Fig-1a]. Further, the contractions produced by 10 µM of ACh (n=6) were significantly blocked by prior exposure of muscle strips to 100 µM of atropine (p<0.05, [Table/Fig-1b,c]).
3. 5-HT Produced Muscle Contractions in Inflamed Vermiform Appendix
A 5-HT (0.1 µM-3 µM) elicited contractions in the longitudinal muscle strips of inflamed vermiform appendix. The muscle contractions elicited by 5-HT demonstrated a dose-dependent increase in amplitude up to 1 µM. The maximum contraction was observed at 1 µM of 5-HT and thereafter, the amplitude of muscle contraction decreased as the dose of 5-HT was increased to 3 µM (n=6 at each dose). The amplitude of contractions obtained at each dose of 5-HT were significantly different (p<0.05, [Table/Fig-2a]).
a) shows dose-response of 5-HT (0.1-3 µM) elicited muscle contractions (g/g) in inflamed vermiform appendix. The values of amplitude of contractions are represented as Mean±SEM (n=6) at each dose. The contractions at each dose are significantly different (p<0.05, One-way ANOVA). An asterisk (*) indicates significant difference among the responses obtained at each dose; b) demonstrates the original recording of an experiment of 5-HT (1 µM) induced contractions before and after exposure to ondansetron (10 µM). Scale for measuring the tension and time are shown at the top left corner; c) demonstrates Mean±SEM value (n=6) of the 5-HT (1µM) elicited contractions (g/g) before and after exposure to ondansetron (10 µM). Muscle contractions produced by 5-HT in longitudinal muscle strips of inflamed appendix were significantly blocked by ondansetron pre-treatment (p<0.05, Student t-test for paired observations). Asterisk (*) indicates significant difference from before values. @ indicates significant difference from before values.
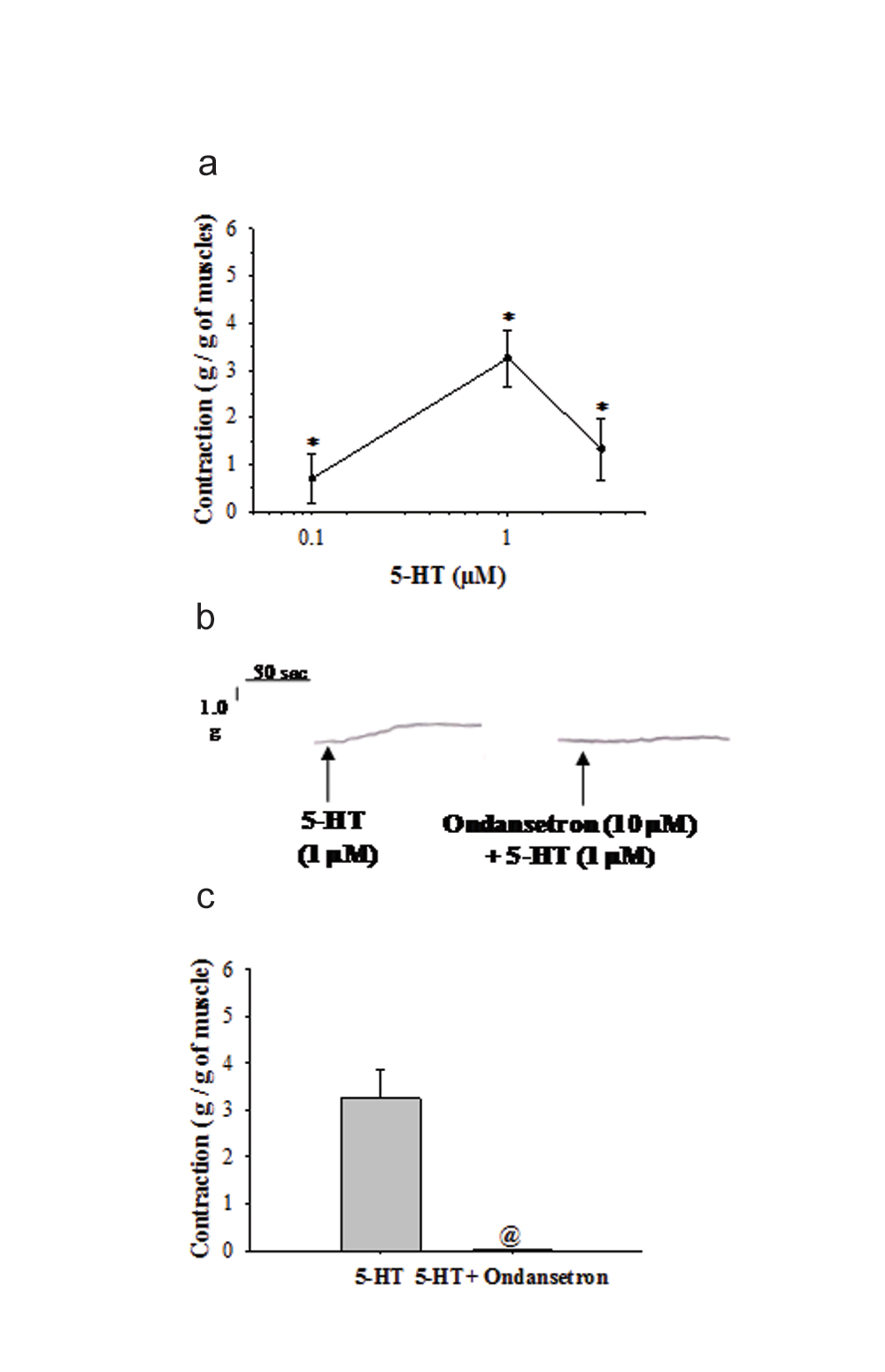
4. 5-HT Induced Contractions were Blocked by Ondansetron
In this group of experiments, the amplitude of contractions elicited by 5-HT (1 µM) were almost same as those observed in dose-response experiments [Table/Fig-2a]. Further, the contractions produced by 1 µM of 5-HT (n=6) were significantly blocked by prior exposure of muscle strips to 10 µM of ondansetron (p<0.05, [Table/Fig-2b,c]).
5. Histamine Induced Feeble Contractions in Inflamed Vermiform Appendix
Histamine (0.01 µM-10 µM) elicited feeble contractions in longitudinal muscle strips of inflamed appendix. Further, histamine did not demonstrate any dose dependent relation in the muscle contractions. The amplitude of contractions were almost equal at each dose (n=6) and the contractions were not significantly different (p>0.05, [Table/Fig-3a]).
a) demonstrates dose-response of histamine (0.01-10 µM) induced muscle contractions (g/g) in inflamed appendix. Values are represented as Mean±SEM (n=6 at each dose). The responses are not significantly different from each other at different doses (p>0.05, One-way ANOVA); b) shows the actual recordings of muscle contraction from individual experiment induced by histamine (10 µM) before and after exposure to CPM (100 µM). Scale for measuring the tension and time scale are given at the top left corner; c) shows Mean±SEM values (n=6) of the histamine induced muscle contractions (g/g) before and after the application of CPM (100 µM). Contractions elicited by histamine in inflamed appendix muscle strips were blocked by CPM pre-treatment (p<0.05, Student t-test for paired observations). @ indicates significant difference from unexposed group.
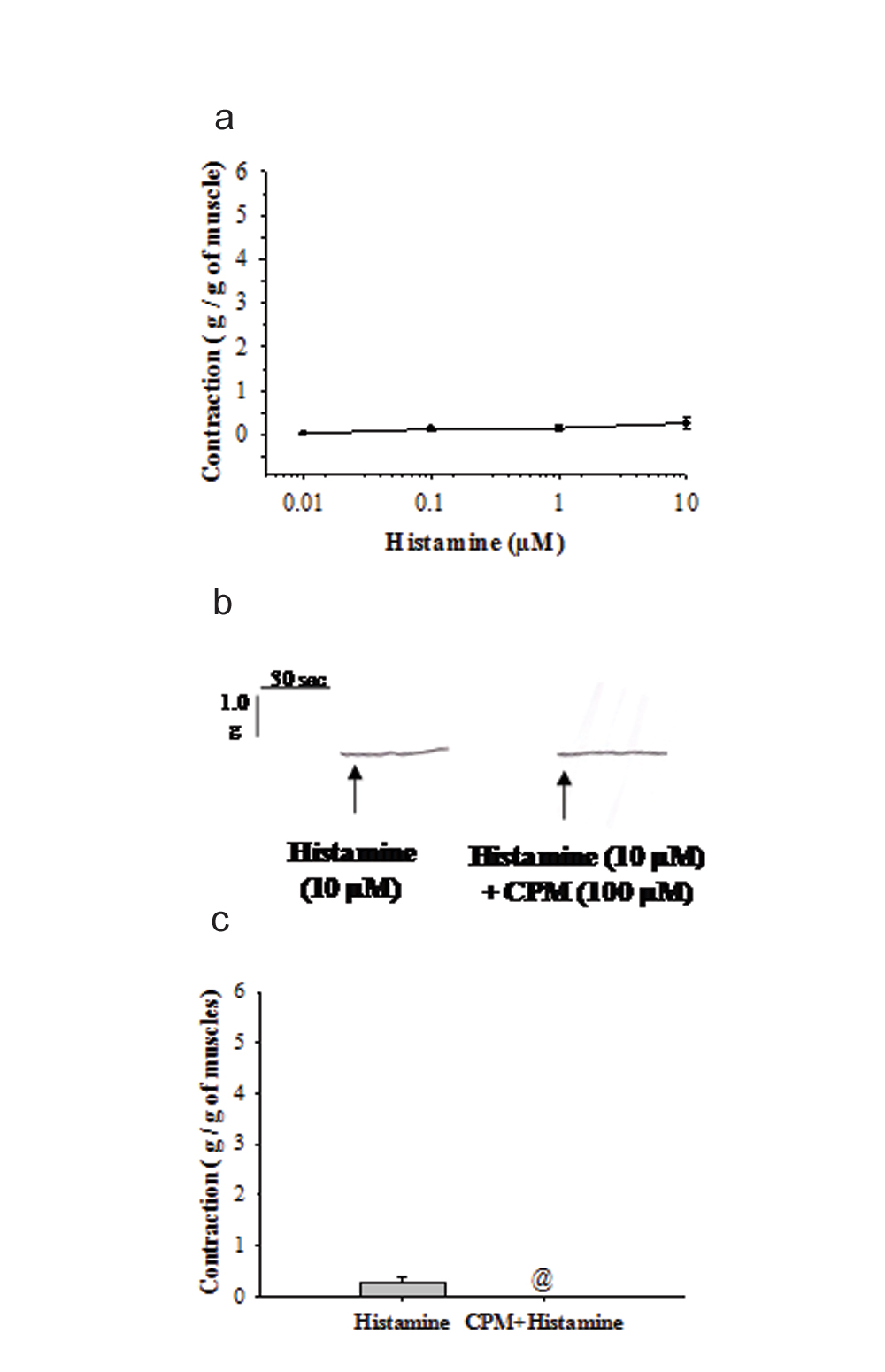
6. Histamine Induced Contractions were Blocked by Cpm
Histamine (10 µM) induced contractions (n=6) were blocked by prior exposure of the tissue to 100 µM of CPM (p<0.05, [Table/Fig-3b,c]). Further, the amplitude of contractions produced by histamine (0.01 µM-10 µM) in inflamed appendix were 14% of ACh (10 µM) and 8% of 1µM of 5-HT ([Table/Fig-1c,2c,3c]).
Discussion
In the present in vitro study, longitudinal muscle strips of inflamed vermiform appendix demonstrated contractile responses to ACh, 5-HT and histamine. Pre-exposure of the muscle strips to atropine significantly blocked ACh elicited responses. Pretreatment with ondansetron completely blocked the 5-HT induced contractile responses. Further, histamine induced contractions were of small amplitude but they were blocked by prior application with CPM.
In the present study, dose-dependent increase in the amplitude of contractions of inflamed appendix observed with ACh (0.01 µM-10 µM) and 5-HT (0.1 µM-1 µM) may be attributed to the increase in sensitivity of the muscle to ACh or 5-HT [Table/Fig-1a,2a]. This may be due to either upregulation of receptors or participation of more and more receptors [8]. In this study, increase in the doses of ACh (100 µM) caused mild decrease in the response and this decrease was prominent when dose of 5-HT was increased up to 3 µM. Diminution in amplitude of the muscle contractions [Table/Fig-2a] at higher dose of 5-HT (3 µM) or ACh (100 µM) may be attributed to the downregulation or inactivation of the receptors [8-10].
Vermiform appendix contains enteric nervous system as it is present in the other parts of gastrointestinal tissues. The myentric Auerbach’s plexus is located between the longitudinal and circular muscle layers. Further extrinsically, myentric plexus is supplied by parasympathetic fibres of vagus nerve [6]. In the present study, ACh produced contractions of longitudinal muscle strips of the inflamed appendix [Table/Fig-1a]. Previous studies demonstrated blockade of electrically stimulated contractions of normal appendix by prior exposure of muscle tissue to atropine, suggesting, cholinergic involvement [11,12]. Further, studies also reported the involvement of muscarinic receptors in mediating the ACh induced contractility in the smooth muscles of gut and normal appendix [5,13]. In the present study, atropine pretreatment significantly blocked ACh induced contractions of longitudinal muscle strips of inflamed appendix [Table/Fig-1c]. Thus, findings suggest the role of cholinergic-muscarinic mechanisms in mediating the contractility in inflamed appendix.
However, ACh induced contractions were not completely blocked by atropine as observed in the present study. The blockade was about 90% which indicates the role of mechanisms other than muscarinic pathways [Table/Fig-1c]. Earlier studies reported the existence of multiple types of interneurons in the enteric nervous system of the gut which receive their innervations by parasympathetic fibers of vagus nerve. Cholinergic activation of these neurons releases variety of neurotransmitters and amines like 5-HT which mediate contractions in the smooth muscles of gut [5,6,14]. Thus, these observations also indicate the role of pathways other than muscarinic for mediating ACh induced contractions in inflamed appendix.
In the present observations, 5-HT produced contractions in longitudinal muscle strips of inflamed appendix [Table/Fig-2a]. Reports suggested that 5-HT mediated its contractile effect in the gastrointestinal system either directly or via myenteric neuronal network [5,6]. Further, the presence of 5-HT was reported in myenteric Auerbach’s plexus and enterochromaffin cells of the vermiform appendix [15]. In this study, ondansetron pretreatment blocked 5-HT induced contractions in the longitudinal muscle strips of inflamed appendix [Table/Fig-2b,c]. Ondansetron has been classified as 5-HT3 blocker [16]. Thus, the findings suggest the involvement of 5-HT3 pathways in mediating the contractility of the inflamed appendix.
Histamine produced contractility of inflamed appendix in the present study [Table/Fig-3a]. Similar observations of histamine are reported in other gut tissue like stomach, colon and even in normal appendix [17,18]. In the present observations, histamine induced contractions in inflamed appendix were significantly blocked by pretreatment with CPM [Table/Fig-3c]. CPM is a known H1- receptor blocker and it mediates contractions of the smooth muscles by activating phospholipase-C in the intestine [6]. Thus, findings suggest the role of H1- receptors in mediating the histamine induced contractility of inflamed appendix.
Further, histamine did not demonstrate any dose-dependent alteration in the amplitude of muscle contractions and contractions were almost of equal amplitude at each dose in this study [Table/Fig-3a]. In addition, amplitude of histamine (0.01 µM-10 µM) induced contractions were lower in comparison to ACh (10 µM) or 5-HT (1 µM) induced contractions in inflamed appendix and the amplitude was 14% of ACh and 8% of 5-HT [Table/Fig-1c,2c,3c]. Above observations suggest the participation of very low population of H1-receptors in contractility of inflamed appendix muscle. Further, observations also suggest that there is no upregulation/ downregulation of receptors on exposure with regard to increased dose of histamine. Thus, findings suggest the minor contribution of histamine in mediating the contractility of inflamed appendix.
On comparing the contractility of inflamed appendix from present study with contractility of normal appendix from earlier observations of this laboratory [5], it was observed that ACh (10 µM) induced contractility in inflamed appendix was reduced by 59%, whereas 5-HT (1 µM) induced contractility in inflamed appendix was reduced by 27% [Table/Fig-4].
Comparison of contractility of normal and inflamed appendix. The data of normal appendix is derived from our earlier observation [5]. Data of inflamed appendix is derived from [Table/Fig-1a,2a] of the present observation (Down arrow (↓) shows diminution in response of inflamed appendix in comparison to normal).
| Agonists | Contractility in normal appendix (g/g) | Contractility in inflamed appendix (g/g) | % change in contractility |
|---|
| ACh (10 μΜ) | 4.28 | 1.75 | ↓59.12 |
| 5-HT (1 μΜ) | 4.47 | 3.25 | ↓27.30 |
Decreased contractility have been reported in other parts of the intestine where any injury that leads to inflammation reduces the motility of the intestine [19,20]. Injury or inflammation has been reported to cause ultrastructural damage to the myenteric neurons [3,4].
Thus, reduction in magnitude of contractions of longitudinal muscle strips of inflamed appendix may be attributed to ultrastructural damage to appendicular muscle myenteric Aurbach’s neurons. Further, varieties of chemicals are released in inflammation. The chemical injury may cause denaturation of receptors and contractile proteins of muscles [20]. Hence, diminution in quantity and quality of receptors or contractile proteins may lead to significant decrease in magnitude of contractions of longitudinal muscles of inflamed appendix as observed in the study. Further, there are reports that cholinergic activation activates neurons that secrete nitric oxide, Vasoactive Intestinal Polypeptide (VIP) and ATP. These substances produce smooth muscle relaxation [6]. Thus, above explanation may be the additional pathway which may produce significant diminution in the magnitude of contraction in inflamed appendix muscle as observed in the study.
In the [Table/Fig-5], atropine (100 µM) pre-treatment increased the blockade of ACh (10µM) induced muscle contractions in inflamed appendix to 88% from 71.5% in normal appendix whereas, blockade by ondansetron pre-treatment was almost equal in normal as well in inflamed appendix. A 5-HT is a mediator released in inflammatory conditions [21]. Thus, the failure of 5-HT to increase the contractility in the inflamed appendix points toward the pathology lying in the muscle itself. However, increase in blockade by atropine demonstrates enhanced role for cholinergic-muscarinic mechanisms in inflamed appendix.
Comparison of effect of antagonist on muscle contractility of normal and inflamed appendix. The data of normal appendix is derived from our earlier observation [5]. Data of inflamed appendix is derived from [Table/Fig-1c,2c] of the present observation.
| Anatagonist + Agonist | Blockade of contraction in normal appendix (%) | Blockade of contraction in inflamed appendix (%) |
|---|
| Atropine (100 μΜ) + ACh (10 μΜ) | 71.5 | 88 |
| Ondansetron (10 μΜ) + 5-HT (1 μΜ) | 98.8 | 99.7 |
Further, there are plenty of neurotransmitters/chemical agents that play important role in mediating the contractility in other parts of the gut [6] but in this study the role of ACh, 5-HT and histamine was investigated. Thus, experiments are required in future to investigate the role of other chemical agents which could enrich the pathophysiology of contractility of inflamed appendix.
Limitation
An important limitation of the study was related to the use of physiograph instead of computerised data acquisition system which could have also recorded the frequency of contractions along with the amplitude of contractions. The present study was performed on longitudinal muscles in in vitro situations. Hence, this study did not provide information that could explain the contractility of the circular muscles of the inflamed appendix. Further, reservations are there to extrapolate the present in vitro findings with in vivo situations of inflamed appendix. However, the positive aspect of the study was that it enhanced the basic pool of knowledge about appendicitis.
Conclusion
The findings suggested that the contractility of the longitudinal muscle strips of inflamed vermiform appendix is decreased in comparison to normal appendix and it is mediated pre-dominantly via cholinergic-muscarinic and serotonergic (5-HT3) mechanisms. Histaminergic (H1) mechanism plays minor role in mediating the contractility in inflamed appendix.