Dental Pulp has an inherent potential to heal and form hard tissue barrier following pulpotomy. This is influenced by the medicament or technique that is used for pulpotomy [1].
Formocresol has been considered the gold standard as pulpotomy medicament and has been extensively used. Since the International Agency for Research on Cancer classified formaldehyde as carcinogenic for humans in June 2004, quest for newer, safer materials have been initiated [2].
Ferric sulfate, a haemostatic agent is one among them. It has been investigated and has been found to be comparable to the gold standard [3].
Laser has also been used for several of its benefits like faster action, ease of use, patient acceptance etc., [4].
Studies have shown that lasers have shown to increase healing, stimulate dentinogenesis and preserve vitality of the dental pulp and is considered a promising alternative for conventional pulpotomy therapy [5,6].
Clinical and radiographic evaluation of pulpotomies using ferric sulfate and laser have been estimated in numerous studies but studying the histologic response of pulp to laser and ferric sulfate will give a better insight into their mechanism of action. Literature search demonstrates many clinical studies exists comparing the laser and ferric sulfate pulpotomy [7-9] and very few that evaluate the histological changes in pulp following laser and ferric sulfate pulpotomy [5,10]. Thus, the aim of the present study was to evaluate and compare histological changes seen in the pulp following ferric sulfate and laser pulpotomy.
Materials and Methods
The present study was a single blind in vivo study, where the oral pathologist was blinded. Institutional Ethics Committee clearance was obtained prior to the study. The study was conducted in the Department of Paedodontics and Preventive Dentistry and department of Oral Pathology, Manipal College of Dental Sciences, Mangalore, Karnataka, India. Only parents and children who signed the consent and accent form respectively were included in the study.
Sample Selection and Distribution
Sample size was calculated using resource equation E= Total number of animals – Total number of groups, where E is should be between 10 to 20. A loss of sample of 20% was considered to estimate the sample size. Total of 24 primary teeth from 22 children in the age group of 7-10 years were selected for the study.
Inclusion criteria for the study were teeth that were indicated for pulpotomy but had less than ½ root remaining and therefore would exfoliate within 4-5 months. This ensured that removal of the experimental tooth would be coinciding with its natural exfoliation time. In the present study, the routine treatment for the selected subjects would have been extraction as the teeth were nearing exfoliation. We did the treatment free of cost explaining all the issues and procedures to the parents.
The teeth that were excluded from the study were those with tenderness to percussion, mobility, fistulation or any signs and symptoms indicating extensive pulpal and periapical involvement and medically compromised children.
The teeth thus selected were randomly divided into two groups based on the material to be used (ferric sulfate and laser) and subgrouped based on the time period (30 and 45 days) of the study [Table/Fig-1]. Lignocaine hydrochloride (2%) with epinephrine (1:2,00,000) (Astra Zeneca, Pharma India Ltd., Bangalore, India) was used to anaesthetize the selected tooth. Following rubber dam application, access cavity was prepared on the occlusal surface of the molars using sterile diamond bur (ISO 001/012 Dia-Burs, Mani Inc, Tochigi, Japan) at high speed under air-water spray coolant. Coronal pulp was amputated using sharp spoon excavator. The bleeding was controlled using sterile cotton pellet soaked in saline. After achieving complete haemostasis, the pulp was exposed to one of the study material.
| Group | Period of study | Sample size |
|---|
| Group 1 (ferric sulfate) | Group IA – 30 days | 6 |
| Group IB - 45 days | 6 |
| Group 2 (laser) | Group IIA - 30 days | 6 |
| Group IIB - 45 days | 6 |
| Total | 24 |
Group A: ferric sulfate pulpotomy: A 15.5% Ferric sulfate solution (Viscostat, Ultradent Products, Inc. US) was used to burnish the pulp for 15 seconds. Pulp chamber was then flushed with water. Zinc oxide eugenol cement was placed over the pulp stump and tooth restored with glass ionomer cement (GC India) [10].
Group B: laser pulpotomy: Pulp stump was irradiated using semiconductor diode laser device (AMD PICASSO 810 nm Diode Laser Kit, Dentsply). The laser was applied in continuous mode for 5 seconds. Laser was delivered through 0.5 mm diameter optical fiber, in contact mode. Zinc oxide eugenol cement was placed over the pulp stump and tooth restored with Glass Ionomer Cement [11].
The teeth were then extracted at the end of the observation period either 30 or 45 days under local anaesthesia (lignocaine hydrochloride (2%) with epinephrine-1:2,00,000).
Preparation of the Extracted Tooth for Histological Analysis
The extracted teeth were immersed immediately in 10% neutral buffered formalin solution and allowed to remain in it for ten days to facilitate fixation. The teeth were then decalcified using 10% formal formic acid (Gooding and Stewart solution). A 100 ml of 85% formic acid was added to 50 ml of formalin and 850 ml of distilled water to obtain the decalcifying solution of desired concentration. The specimens were immersed in the solution for a period of fivedays and the demineralization was checked using radiographs.
Tissue Processing: The decalcified specimens were left immersed in running water for 24 hours to ensure complete removal of the formic acid. The specimens were subjected to routine histological tissue processing and the processed specimens were embedded in molten paraffin wax. Serial sections of 5 µm were cut using soft tissue microtome (Reichart-Zung, Cambridge instruments, West Germany) in a buccolingual plane. The tissue sections were mounted on gelatin coated slides and subjected to Haematoxylin and Eosin staining (H&E staining) procedure.
Evaluation of the Specimens: The H&E stained slides were evaluated for pulpal response to the pulpotomy agent with respect to dentin bridge formation, quality of dentin formation in the bridges, location of dentin bridges, tissue reaction to the material, inflammatory cell response and necrosis with the help of optical microscope. The evaluation was done according to the criteria adapted from a modified scoring system [Table/Fig-2] [12].
Modified evaluation criteria used for the assessment of pulpal response.
| Dentin bridge formation | Quality of dentin formation in the bridge | Location of dentin bridge | Tissue reaction to the material | Inflammatory cell response | Necrosis |
|---|
| Score 0 | No bridge formation | No tubules present | At the interface of exposed pulp | No macrophages/giant cells adjacent to the material | No scattered inflammatory cells in the pulp area corresponding to the pulpexposed characteristic tissue. | Absent |
| Score 1 | Interrupted bridge formation | Irregular pattern of tubules | Not at the interface of exposed pulp | Mild infiltration of macrophages/ giant cells. | Slight inflammatory cell infiltrate with PMNs or MNLs. | Denaturation of proteins, autolysis |
| Score 2 | Continuous bridge formation | Regular pattern of tubules | Combination | Moderate infiltration of macrophages/ giant cells. | Moderate inflammatory cell infiltrate involving the coronal third of the radicular pulp. | -- |
| Score 3 | -- | -- | -- | Severe infiltration of macrophages / giant cells. | Severe inflammatory cell infiltrate involving the coronal third of the radicular pulp. | -- |
Statistical Analysis
The statistical analysis was done using SPSS 20.0 software. Intragroup comparisons of the observed values were analysed using Chi-square test.
Results
None of the children showed any clinical signs and symptoms indicating failure such as pain, swelling or fever during the observation period.
Tissue changes were observed under microscope (Magnification of x100) [Table/Fig-3,4,5,6 and 7].
Results of analysis of the hard tissue barrier.
| Parameters | Dentin bridge formation | Quality of dentin bridge | Location of dentin bridge |
|---|
| 30 days | 45 Days | 30 days | 45 Days | 30 days | 45 Days |
|---|
| Scores | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 |
| Ferric sulfate | 3 | 3 | - | - | 6 | - | 2 | 1 | - | 2 | 4 | - | 3 | - | - | 4 | 2 | - |
| Laser | 2 | 3 | 1 | 1 | 4 | 1 | - | 4 | - | - | 4 | 1 | 3 | 1 | - | 5 | - | - |
| Level of Significance p<0.05 | p=0.375 | p= 1.000 | p=0.143 | p=0.102 | p=0.571 | p= 0.273 |
Results of Pulpal Response.
| Parameters | Tissue reaction | Inflammatory cell response | Necrosis |
|---|
| 30 days | 45 Days | 30 days | 45 Days | 30 days | 45 Days |
|---|
| Scores | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 0 | 1 |
| Ferric sulfate | 1 | 1 | 4 | - | 2 | 4 | - | - | 1 | 1 | 4 | - | 2 | - | 4 | - | 6 | - | 6 | - |
| Laser | 2 | 3 | 1 | - | 0 | 4 | 1 | - | 3 | 1 | 1 | - | - | 4 | 2 | - | 6 | - | 4 | 2 |
| Level of Significance p<0.05 | p=0.079 | p= 0.317 | p=0.215 | p= 1.000 | p=0.227 |
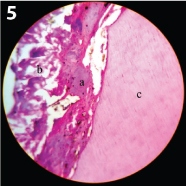
A Continuous dentin bridge seen in the ferric sulfate treated sample (45 days sample) (100x magnification) a) Continuous bridge: b) Pulp tissue; c) Dentin.
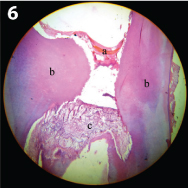
Continuous tubular dentin bridge seen in the laser treated sample (45 days sample) (100x magnification) a) Bridge; b) Dentin; c) Pulp tissue.
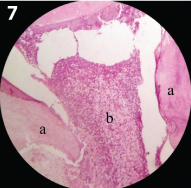
Inflammatory cell infiltration seen in the ferric sulfate treated sample (45days sample) (10x magnification) a) Dentin: b) Inflammatory cells in the Pulp tissue.
Hard tissue was analysed for dentin bridge formation, quality and location of the dentin bridge formed
Dentin Bridge Formation
The surface of the exposed pulp was analysed for dentin bridge formation. By 45 days, all samples in ferric sulfate group showed interrupted bridge formation but this was not so in the laser group.
The difference in the ability of the materials to produce a reparativedentin bridge in 30 days and 45 days was found to be statistically non-significant (p=0.375 and p=1.00 respectively).
Quality of Dentin Bridge
The reparative dentin that was formed was analysed to determine the quality of the bridges. Fifty percent of the samples in ferric sulfate group showed irregular tubular formation by 45 days and remaining had no tubular structure, whereas one sample in the laser group exhibited regular pattern of tubules.
Statistical analysis using Chi square test revealed non-significant difference (p=0.143 and 0.102) in the quality of dentin bridges formed in two groups during both the observational periods.
Location of Dentin Bridge
Majority of the samples in both ferric sulfate and laser group exhibited dentin bridge at the interface of the exposed pulp, bridging or attempting to bridge the site exposed to the pulpotomy material. Statistical analysis revealed non-significant difference (p=0.571 and 0.273).
Tissue Reaction
Laser group showed more macrophage infiltration compared to ferric sulfate group. This difference in the cellular reaction between the groups was not significant (p=0.079 and 0.317).
Inflammatory Cell Response
Inflammatory cell infiltration increased as the days increased in both the groups, except in two samples of ferric sulfate which showed no signs of infiltration in 45 days group.
The difference between the groups in evoking an inflammatory cell response was statistically non significant (p=0.215 and 1.000).
Necrosis
Necrosis was identified based on the denaturation of proteins and autolysis of the tissue adjacent to the capping material. All the samples of both the groups showed no signs of necrosis in 30 day group and two samples of laser group showing severe necrosis in 45 days period. The difference between the groups in 30 days group was statistically significant (p<0.001).
Discussion
The response of the pulp to a material is the best criteria to judge the effectiveness of a medicament used for pulpotomy [13].
Ferric sulfate has been proposed as an alternative for formocresol. It is a non-aldehyde haemostatic agent believed to minimize the chances of inflammation and internal resorption. Ferric sulfate produces a ferric ion-protein complex when it comes in contact with blood and this seals the cut vessels mechanically, producing haemostasis and preventing the formation of a blood clot [14,15].
Results have been more favorable with ferric sulfate when compared to formacresol for the purpose of pulpotomy [16-18].
Use of laser for pulpotomy has many advantages such as control of haemorrhage, sterilization of the lesion, stimulation of dental pulp cells causing increased healing, dentinogenesis and preservation of vitality of the dental pulp. It has been therefore proposed as an alternative for pulpotomy of primary teeth, due to its advantages such as control of hemorrhage, sterilize the lesion, stimulate dental pulp cells causing increased healing, dentinogenesis and preservation of vitality of the dental pulp [19].
Nd YAG laser, CO2 or diode lasers have been recommended for pulpotomy [7]. Diode lasers have been used widely in soft tissue oral surgery procedures. These lasers are relatively poorly absorbed by the tooth structure and thus soft tissue surgery can be performed safely on soft tissues such as pulp that are close to enamel and dentin [7,8].
Laser causes an instant and reversible decrease in blood flow for 3–6 minutes without any hyperemic reaction in the pulp microcirculation. This laser-induced haemorrhage can mask the true hyperemia in the radicular pulp, which can be mentioned as one of the disadvantages with laser [20].
Ideally, when pulp tissue is treated with laser irradiation a superficial zone of coagulation necrosis is created that is compatible with the underlying tissue and isolates the pulp from the harmful effects of the subbase. Variation in laser application parameters, including the power, frequency, exposure time, and water/air dry-mode, yields different results in pulp tissue. These facts might be responsible for the conflicting results in the laser-assisted pulpotomy cases [8].
In the present study, 810 nm diode laser was delivered onto the pulp stump at 1.4 W in continuous mode for 5 seconds in contact mode until the tissue was seen to be ablated. Thermal injury to the pulp depends on the length of laser exposure rather than the output power of the device [11].
Hard Tissue Barrier
Interrupted dentine bridge was formed in all six samples of the ferric sulfate group at the end of 45 days as compared to four samples of the laser group. Cleaton. Jones P et al., evaluated the histologic response of pulp to ferric sulfate in primary teeth in baboons [21] and found that inflammation is followed by repair and formation of reparative dentin is part of the repair process that occurs whenever there is injury to the pulp [22].
One sample each from the laser group showed continuous dentin bridge formation at the end of 30 days and 45 days. Thus, it can be established that laser induced dentin bridge formation is comparable to ferric sulfate. It appears that over a period of time, dentin bridge forms in response to both ferric sulfate and laser.
It should also be noted that the presence of atubular dentin has been found to be important as it provides a “barrier effect” against penetration of noxious agents [23]. Few authors have also speculated that the initially formed atubular dentin or osteodentin can become progressively lined with tubules forming tubular dentin over a period of time [24,25].
But this result has not been observed in our study especially the ferrous sulfate group as no samples showed tubular dentin in 45 days period. One sample in laser group demonstrated tubular dentin.
Tissue Reaction
Mild infiltration of macrophages or giant cells was seen in four samples of both the ferric sulfate and laser group at the end of 45 days. Similar response was seen in the study by Odabas ME et al., in the 60 days group of teeth irradiated with Nd:YAG laser [26].
The appearance of macrophages has also been attributed to be a sign of healing as they play an important role in scavenging superficial necrotic tissues [13]. Macrophages increased in ferric sulfate group upto 30 days and by 45 days there was a fall. But in laser group there was a steady rise in macrophage count throughout the study.
Moderate inflammatory cell infiltration were seen more in the ferric sulfate than in the laser groups This shows that the inflammation is less in laser treated teeth as compared to the teeth treated with ferric sulfate. Elliott RD et al., saw similar results in a study where the pulp was treated with carbon dioxide laser [27]. Same or mild decrease in inflammatory cells was seen in ferric sulfate group while there was a mild rise in laser group by 45 days.
Two samples in the 45 day laser group showed necrosis whereas, none of the other samples showed necrosis. Shoji S et al., investigated the immediate effects of a CO2 laser on amputated dental pulps in dogs [9]. Histologically, they found a thin layer of coagulation necrosis, coagulation, and degeneration of the odontoblastic cell. Such extensive damage was not observed in the current study supposedly because of the variation of the laser and the settings used to perform the pulpotomy procedure. In another study by Mareddy A et al., diode laser 810 nm was used in pulpotomy of dog’s teeth in which the necrosis was limited to the site of exposure similar to our study [11].
As observed from the literature, the overall clinical success of ferric sulfate pulpotomy from the studies ranged from 78%–100% while the overall radiographic success varies from 43%-97% [19]. For the laser pulpotomy the clinical success ranged from 85.71% –100% while the radiographic success varied from 67%–94.1% [22].
Yadav P et al., found the overall clinical and radiographic success of ferric sulfate was 86.6% and 80% respectively and 100% and 80% for the diode laser group, with no significant difference between any group at the end of nine months [28].
In a study by Gupta G et al., ferrous sulfate was found to be less effective than laser with 20% clinical failure. They attributed the clinical success of laser pulpotomy to its non-invasive and non-pharmaceutical nature of technique [29].
Histologically, the results were found to be comparable, where laser group showed a better result in the 30 day group as compared to ferric sulfate group with dentin bridge formation. Even though laser samples exhibited lower success rate as compared to the ferric sulfate group at the end of 45 days, dentin formed in a sample of the 45 day laser group showed organized dentinal tubules which was not exhibited by any of the samples in the ferric sulfate groups. Inflammatory response and tissue response were similar in both groups.
Thus, it can be summarized that laser can induce interrupted dentin bridge formation by the end of 30 days. It can be proposed that laser is able to induce continuous dentin bridge with organized dentinal tubules by the end of a longer time period with milder tissue reaction. Thus, making it a better non pharmacologic alternative to ferric sulfate.
Clinical implications: Laser is advantageous as it aids in adequate healing of the tissues with minimal bleeding and can be considered as an effective alternative for pulpotomy in primary teeth.
Limitation
The present study compares only two pulpotomy agents. Therefore future scope of the study would be to evaluate different types of soft tissue lasers when used for pulpotomy.
Conclusion
The inference that could be derived from the present study is that Laser and ferric sulfate are capable of producing dentin bridge at the interface between the pulp and the material but the quality of bridge formed was better in laser group. Laser group also showed more macrophage infiltration, inflammatory cell infiltration and areas of necrosis compared to ferric sulfate group though not statistically significant.