Cancer of the head and neck involves a gathering of neoplasms that share a comparative anatomical origin. Head and neck cancer rank 6th in overall neoplasms of all areas and the most ordinarily influenced site is the oral cavity [1]. It has been figured that roughly 400,000 new cases per year are analysed around the world, with higher predominance in males. Oral cavity and the oropharynx being the most common sites for occurrence of OSCC.
HPV is a double-stranded DNA virus and, to date, the sequences of over 200 types have been described [2]. HPV16 is the most prevalent in cancers among the HPV serotypes. The role of the HPV has now been perceived as an independent factor in the development of these cancers. Squamous cell carcinoma associated with HPV has its frequency increasing in young patients and in non smokers or drinkers, especially associated with high risk serotypes such as viral 16 subtypes. This has been supported by reports in the literature which indicates that HPV is the primary source of oropharyngeal squamous cell carcinoma [3,4]. High risk serotypes of HPV that are associated with cancer of the oral cavity and oropharynx are 16 and 18, whereas serotypes 6 and 11 are regarded as low risk and have been linked to cancer of the larynx [5]. Recognition of HPV is completed by different techniques out of which two well-known are PCR and IHC.
PCR, developed in 1983 by Karey Mullis, who also won the noble prize for this invention, led PCR to become a central technique in biochemistry and molecular biology [6]. The technique allows prompt detection of low abundance viruses and identification of a single genome copy. Use of PCR in Pakistan may be limited as a confirmatory tool in reference laboratories and donor screening in blood banks because of its cost and technical inadequacy [7]. PCR has shown positive results during the detection of pulmonary and extrapulmonary tuberculosis, having a potentially important role in rapid diagnosis of TB and proving to be more sensitive and highly specific when compared to commonly use conventional techniques [8]. The procedure has its benefits; it can be performed in a single day, easily regulated and therefore, considered as highly sensitive and practical. For cytological samples and for the detection and typing of HPV genomes in biopsy, it is considered as reliable diagnostic tool. For over 20 year, PCR-based HPV tests have been in use in research having an advantage of providing type specific information [9].
On the other hand, IHC is the use of monoclonal and polyclonal antibodies for the recognition of specific tumour antigens expressed de novo in tissue sections. It is used by many pathologists for diagnosis of different medical issues. A HPV IHC is a technically simple and widely accessible test, however, not all the antigens are equally preserved and detectable through IHC and it is less sensitive and specific than PCR based molecular diagnostic methods [10]. It is a cost-effective technique and also used to recognize distribution of tissues in an antigen of interest in diseases. In assessing HER2 status in clinical specimens, IHC is still the most broadly used method to date. There have been debates about its relative merits for use in various tumours [11]. The technique is utilized as a part of detection for cell or tissue antigens (amino acids, proteins and infectious agents) [12]. It comprises of two phases: (a) slide preparation and stages evolved for the reaction; (b) interpretation and quantification of the obtained expression. IHC is still not believed to be the most sensitive laboratory test for diagnosis of different cancers but it is widely used due to its low equipment and setup cost.
Materials and Methods
This study was based on samples retrospectively collected from 47 patients with primary OSCC who were diagnosed and treated at The Aga Khan University Hospital during the period of January 2010 to Dec 2013. Clinicopathologic and demographic data was obtained from patient files. Prior to enrolment, the patients were informed about the purpose of the study and the necessity for obtaining related information. Patients willing to participate were requested to fill in a questionnaire.
All Haematoxylin and Eosin (H&E) stained slides were reviewed and representative paraffin embedded tissue blocks of selected patients were collected.
DNA Extraction
At least five, 10 µm thick tissue sections from each paraffin embedded block were cut on microtome and placed in 2 ml microcetrifuge tubes. Specimens were deparaffinized with xylene and washed in serial graded ethanol (100%, 90%, 70%, and 50%), then finally washed in Diethylpyrocarbonate (DEPC) treated autoclaved water. Pellets were dried and homogenized with cell lyses buffer containing 400 mM tris-HCl (pH 8.0), 60 mM Ethylenediaminetetraacetic Acid (EDTA), 150 mM NaCl and 1% Sodium Dodecyl Sulfate (SDS). After homogenization 10 mg/ml proteinase K was added and the mixture was incubated at 55ºC overnight to allow digestion by proteinase K.
The following morning, after centrifugation supernatant was collected and 650 µl DNAzol added, after 3 minutes, 500 µl absolute ethanol was added for the precipitation of DNA solution which was then centrifuged and pellets were washed with 70% ethanol and re-suspended in TE buffer, resulting DNA solution was stored at -80ºC until further use.
Polymerase Chain Reaction (PCR)
PCR technique was optimised according to published literature [15]. [Table/Fig-1] depicts the primer for PCR.
Primer used for PCR amplification.
| Primer | Sequence | Target gene | Amplimer length |
|---|
| GP5 | TTTGTTACTGTGGTAGATAC | L1 | 155 bp |
| GP6 | GAAAAATAAACTGTAAATCA | | |
| TS16-A | GGTCGGTGGACCGGTCGATG | L1 | 96 bp |
| TS16-B | GCAATGTAGGTGTATCTCCA | | |
| TS18-A | CCTTGGACGTAAATTTTTGG | L1 | 115 bp |
| TS18-B | CACGCACACGCTTGGCAGGT | | |
| PC03 | ACACAACTGTGTTCACTAGC | β -Globin | 110 bp |
| PC04 | CAACTTCATCCACGTTCACC | | |
Quality Controls
The quality controls used in this study were the same as in the published literature [15].
Immunohistochemistry
A 3 µm-4 µm thick paraffin embedded tissue section were cut and mounted on histogrip (Zymed Laboratories Inc.) coated slides, dried at 56ºC for 30 minutes. Specimens were deparaffinized with xylene, rehydrated in serial graded (100%, 90%, 70%, and 50%) water ethanol solutions and rinsed in deionized water. Target retrieval were performed with target retrieval solution (Dako, Denmark) in preheated (90ºC-95ºC) water bath for 20 minutes. After antigen retrieval endogenous peroxidase activity was blocked by immersion of slides in peroxidase block solution (0.03% hydrogen peroxide containing sodium azide, Dakocytomation, Denmark) for 10 minutes. After washing with TBST (Tris Buffer saline with Tween 20, Dakocytomation, Denmark) sections were incubated with primary antibody against HPV (mouse monoclonal anti-HPV, clone K1H8 DAKO, diluted 1:50) for 30 minutes at room temperature. These were then rinsed in TBST buffer. Afterward, sections were treated with labelled polymer (labelled polymer-HRP anti-mouse) and bound antibody was detected using the polymer technology EnVision + system-HRP (DAB), (Dako, Denmark), according to manufacturer’s instruction. A light Hematoxylin nuclear counter stain was used for nuclear staining. After counter stain specimens were dehydrated in serial graded (50%, 70%, 90%, 100%) water-ethanol solutions, and mounted with DPX reagent and observed under the light microscope. One positive and one negative control were run with each batch of immunostained sections.
Evaluation of Slides
Slides were examined for the presence of brown nuclear staining within the tumour. Result were scored as negative (–) and positive (+). Two histopathologist were included in this study, who were working independently (unaware of each other’s results) to review results of IHC for HPV positivity. Both results were reviewed and were similar.
Statistical Analysis
Chi-Square was used in order to check the association between the Polymerase Chain Reaction and Immunohistochemistry.
Results
The gender based division of the patients is shown in [Table/Fig-2]. Of the 47 evaluated patients, 32 (68.1%) were found positive for HPV; these patients were detected positive only via PCR. IHC failed to detect any positive results for the presence of HPV in these patients [Table/Fig-2]. Among these positive patients detected by PCR, 25 (78.1%) were male and 7 (21.9%) were female. HPV type 16 was more prevalent as it was detected in 27 (57.4%) patients whereas, type 18 was found positive in only 1 (2.1%) patient. Patients were treated with different procedures after the recurrence of disease. Recurrence was noted in 22 (46.8%) patients and 15 (31.9%) patients were reported negative for any recurrence [Table/Fig-2].
Demographics of patients.
| Characteristic | Numbers | % | HPV + (%) | HPV – (%) | p-value |
|---|
| Age Division |
| >40 Years | 39 | 82.97 | 28 (59.6) | 11 (23.4) | 0.63 |
| <40 Years | 8 | 17.02 | 5 (10.6) | 3 (6.4) |
| Gender |
| Male | 32 | 68.08 | 25 (53.2) | 7 (14.9) | 0.03 |
| Female | 15 | 31.91 | 7 (14.9) | 8 (17.0) |
| Histological Type |
| PDSSC | 25 | 53.19 | 15 (31.9) | 10 (21.3) | 0.44 |
| WDSCC | 22 | 46.80 | 17 (36.2) | 5 (10.4) |
| T Classification |
| T1 | 11 | 23.40 | 7 (14.9) | 4 (8.5) | 0.66 |
| T2 | 21 | 44.68 | 16 (34.0) | 5 (10.6) |
| T3 | 6 | 12.76 | 4 (8.5) | 2 (4.3) |
| T4 | 6 | 12.76 | 4 (8.5) | 2 (4.3) |
| Not Mentioned | 3 | 6.38 | 1 (2.1) | 2 (4.3) |
| N Classification |
| N0 | 30 | 63.82 | 22 (46.8) | 8 (17.0) | 0.38 |
| N1 | 10 | 21.27 | 7 (14.9) | 3 (6.4) |
| N2a | 1 | 2.12 | 1 (2.1) | 0 (0) |
| N2b | 3 | 6.38 | 1 (2.1) | 2 (4.3) |
| Not Mentioned | 3 | 6.38 | 1 (2.1) | 2 (4.3) |
| M Classification |
| M0 | 43 | 91.48 | 31 (66.0) | 12 (25.5) | 0.13 |
| M1 | 1 | 2.12 | 0 (0) | 1 (2.1) |
| Not Mentioned | 3 | 6.38 | 1 (2.1) | 2 (4.3) |
| Primary Site |
| Tongue | 14 | 29.78 | 8 (17.4) | 6 (13) | 0.60 |
| Cheek | 29 | 61.70 | 21 (44.7) | 8(17) |
| Palate | 1 | 6.38 | 1 (2.2) | 0 (0) |
| Not Mentioned | 3 | 2.12 | 2 (4.3) | 1 (2.2) |
| Positivity of HPV IHC |
| Positive | 0 | 0.0 | | | |
| Negative | 47 | 100 | | |
| HPV Type Specificity |
| 16 positive | 27 | 57.4 | 27 (57.4) | 0 (0) | <0.001 |
| 18 positive | 1 | 2.1 | 1 (2.1) | 0 (0) |
| 16 and 18 both positive | 3 | 6.4 | 3 (6.4) | 0 (0) |
| Other than 16 and 18 | 1 | 2.1 | 1 (2.1) | 0 (0) |
| Negative | 15 | 31.9 | 0 (0) | 15 (31.9) |
PDSSC: Poorly differentiated squamous cell carcinoma WDSSC: Well-Differentiated squamous cell carcinoma
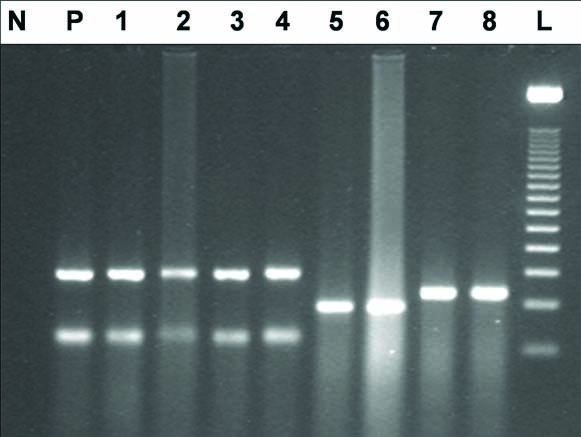
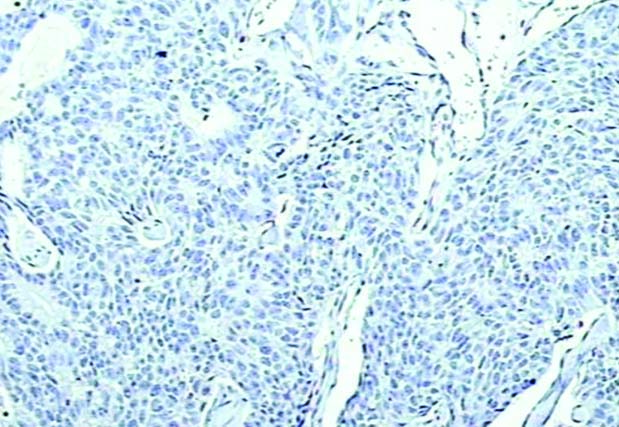
[Table/Fig-3,4] shows the PCR amplification of the HPVs and a negative IHC for a well differentiated OSCC, respectively.
PCR amplification of HPV general, HPV type 16 and HPV type 18 in OSCC samples. The products were electophoresed on 2% agarose gel and stained with ethidium bromide. Lane N: negative control, lane P: positive control, lanes 1-4 HPV (general primer) positive tumour samples, lanes 5-6 HPV 16 positive tumour samples, lanes 7-8 HPV 18 positive tumour samples, Lane L: molecular size marker (50-bp ladder marker).
Photomicrograph of a well differentiated oral Squamous cell carcinoma demonstrating negative HPV immunohistochemistry staining. Magnification X20.
Discussion
Among the techniques available for detection of HPV in tumours, conventional PCR is known to be incomparably the most sensitive of all [16]. This is supported by the results from this study as PCR Detected 32 HPV positive patients when compared to IHC (no positive detection), proving to be highly sensitive. It has the benefit of providing type specific diagnosis, and is being used for more than 20 years now [9], as it can detect HPV well below one viral copy genome per cell. Similarly, PCR properly differentiated the HPV subtypes detecting type16 in majority of patients 57.4% and type18 in only 2.1%. The results support the literature and show the ability of PCR for providing type-specific diagnosis. A study published by Agoston ES et al., in 2010 recognized PCR testing as a credible method of HPV detection from paraffin embedded samples of oropharyngeal squamous cell carcinoma; and this is also comparable with others reported in the literature [17].
Moreover, PCR technique can also be used to assess viral load of HPV in the tumours as quantifying it can be crucial in deciding whether HPV positive oral cancers are unquestionably the result of HPV infection [18]. Real time PCR has, in fact, been regarded as the gold standard for the estimation of HPV viral load [19], having the ability to distinguish HPV infections that are clinically significant from those that are not. This gold standard method involves transcriptional activation of the viral oncoproteins E6 and E7. However, E6/E7 mRNA trials are now being used which are being made easier and transferrable to the diagnostic laboratory, maintaining the same accuracy and reliability.
Sensitivity however, is also a limitation as HPV sequences might be missed by the L1 primers [20]. But there is little evidence that such mechanism is responsible for a large number of false-negative outcomes [21]. This method is more cost-effective than in situ hybridization and can be performed in any diagnostic molecular laboratory, which is why it’s important to determine the sensitivity of this method relative to others. Currently, PCR allows the greatest sensitivity and amplification of HPV subtypes can be done by the use of consensus primers in this process [22].
IHC is another method used commonly for detection of head and neck tumours. Even though it’s use is prevalent, it was shown in a recent study that due to poor concordance between p16 and HPV, p16 should not be used as a surrogate marker for this infection in Head and neck squamous cell carcinoma [23]. Isolated use of p16 IHC can help in identifying tumours with excess p16 protein [24], however its combined use with PCR can improve its specificity as a test, allowing it to be classified into one of four groups [25], depending on a score for the 2 components. When PCR is not feasible, the combination of in situ hybridization with IHC for HPV DNA analysis can also be helpful [26].
Correspondingly, Pannone G et al., affirmed that IHC alone is not a reliable method for detection of HPV in oral cancers [27]. When compared to in situ hybridization, IHC was more inferior with detection rate of 14% (20/140) compared to 61% (86/140) for the in situ assay with the digoxigenin-labeled probes for detecting HPV [28]. Comparative results are concluded from this study too, which shows IHC to be inferior to other techniques (PCR in this study) when detecting HPV as IHC failed to diagnose positivity of HPV in any patient giving insignificant results. Another study stated that HPV DNA-negative but p16-immunopositive cases are actually HPV-negative, based on genetic profiling. It was revealed that the survival rates of these patients was the same as that of HPV-negative patients, which indicated the significance of testing for HPV DNA along with immunostaining to check for HPV positive oral cancers [29]. Similarly, one more study showed p16-IHC/DNA qPCR to be more sensitive and specific (97% and 94% respectively) as compared to RNA qPCR, proving to be the best contradistinguishing test for a suitable outcome. However, since testing for DNA can be quite complicated in actual, detecting p16 overexpression is still preferred when carrying out clinical experiments.
We tested this observation by experimenting IHC on cases which had been identified via PCR to check if the HPV data obtained through IHC was in concordance or not. Analysis of a study conducted in Liverpool showed that a few of the HPV positive cases were negative and some of the HPV-negative samples turned out to be positive when tested with p16 IHC. This false-negative result shows decreased sensitivity of IHC towards detection of HPV status. All the cases tested by p16 IHC in our research turned out to be negative.
Limitation
A small sample size of only 47 was used for the study. A larger sample would increase the reliability of the results. Two totally different techniques were used. Only one antibody clone was used in IHC, better results could be obtained by using two different clones.
Conclusion
According to this research, PCR has proved to be more sensitive than IHC in detecting HPV in OSCC. Due to insignificant diagnosis and unreliable results from IHC and successful detection from PCR we can conclude that PCR, should be the first initial diagnostic test and favored over IHC when detecting HPV among patients.
We acknowledged the call for analysis of DNA in future which would reveal the alterations which have taken place in the DNA sequence, and therefore mutations can be determined. This could serve as the best diagnostic sign as identified by several studied quoted above, which justify the importance of DNA sequencing through PCR in tumour development.
PDSSC: Poorly differentiated squamous cell carcinoma WDSSC: Well-Differentiated squamous cell carcinoma