People Living with HIV (PLHIV) are at an increased risk of developing malignancies. Plasma cell disorders are also reported with increased incidence in PLHIV compared to the general population. Here, we describe three different plasma cell disorders among PLHIV and highlight their atypical presentations and outcomes in these subjects.
Highly Active Antiretroviral Therapy (HAART) has considerably reduced mortality, improved the survival and quality of life of PLHIV and long term morbidities especially cancers have now become major health concerns in them. PLHIV are at an increased risk of many malignancies including plasma cell disorders [1]. The average age, at which PLHIV present with plasma cell disorders is lower than that of the general population. These patients are also likely to present with more atypical findings and have a variable clinical course.
We present a series of three cases of different plasma cell disorders in PLHIV and review the literature.
Case 1
A 50-year-old male, known case of HIV infection and tubercular lymphadenitis, was receiving Antituberculosis Treatment (ATT) and ART(zidovudine/lamivudine/efavirenz). Two months into his six month short course of ATT, the patient presented with progressively increasing shortness of breath and easy fatigability. He had severe pallor and the respiratory examination revealed a right sided pleural effusion. The rest of the physical examination was unremarkable. On investigations, haemoglobin was 4.5 gm/dl with peripheral blood smear showing macrocytic to normocytic anaemia with occasional atypical cells (2% blasts, 5%myelocytes and metamyelocytes) and platelets were diminished. The ART was modified from zidovudine to stavudine based regimen and packed cell transfusions were given. The blood urea was 57 mg/dl, serum creatinine: 1.0 mg/dl and serum protein: 7.1 gm/dl and albumin: 2.1 gm/dl. A chest X-ray showed mediastinal widening and right sided pleural effusion. Pleural fluid analysis revealed: total protein: 4.5 gm/dl, total count: 175 cells/mm3, 60% lymphocytes, 40% polymorphs, Adenine Deaminase (ADA): 17 IU.
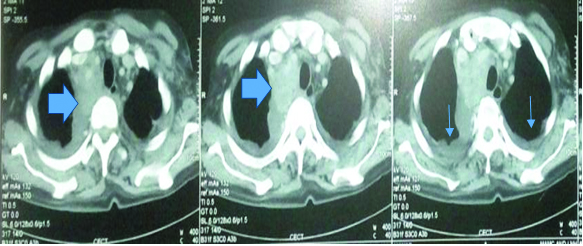
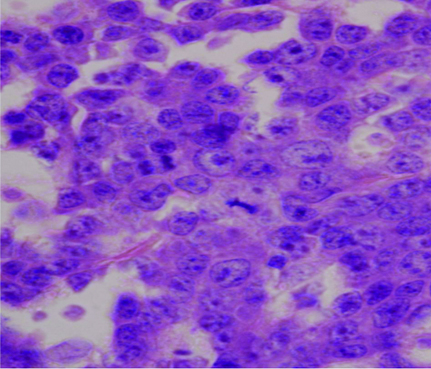
A repeat CD4 count was 47cells/mm3. A Contrast Enhanced Computed Tomography (CECT) of the chest and abdomen revealed a large, irregular, heterogeneous, mass in anterior and middle mediastinum obstructing the bronchus, superior vena cava and associated vascular structures with bilateral pleural effusion and pericardial effusion [Table/Fig-1]. The urine sample for Bence Jones proteins was negative and on serum electrophoresis, an M spike was seen in gamma globulin region. The β2-microglobulin was also elevated: 6.4 mcg/ml (normal range 1.2-2.5 mcg/ml). On bone marrow biopsy examination, the marrow was hypercellular and showed near total replacement by plasmablasts and plasma cells which were present in sheets [Table/Fig-2]. Only a few myeloid and erythroid cells were noted in focally preserved areas. The biopsy taken from the mediastinal mass showed sheets of monoclonal plasma cells suggestive of plasmacytoma. A diagnosis of plasmablastic myeloma was made. The patient however succumbed to his illness prior to institution of definitive treatment.
Contrast enhanced computed tomography (CECT) of the chest re-vealing a large, irregular, heterogeneous, mass in anterior and middle mediastinum obstructing the bronchus, superior vena cava and associated vascular structures with bilateral pleural effusion. (Arrow highlighting the mediastinal mass)
Bone marrow biopsy revealing hypercellular marrow and near total replacement by plasmablasts and plasma cells present in sheets.
Case 2
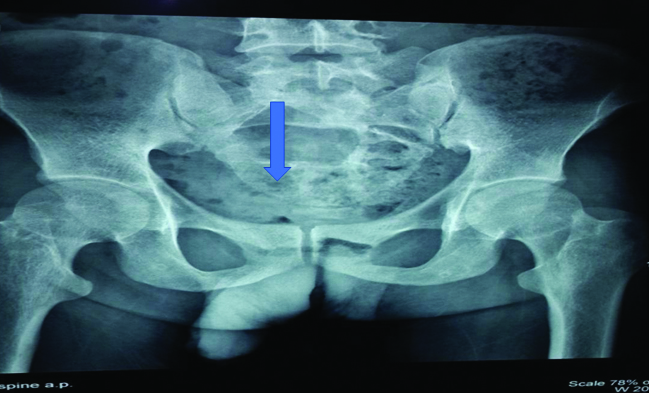
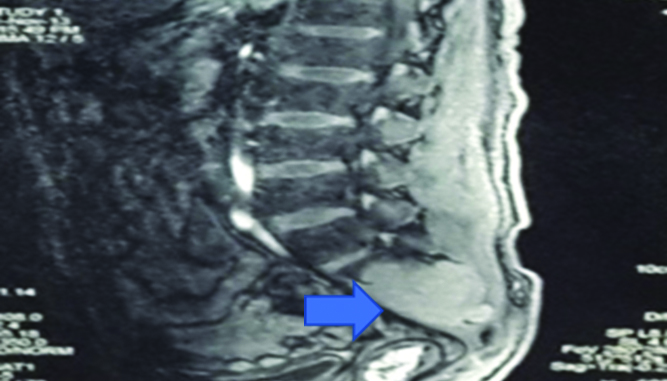
A 33-year-old HIV infected male on first line ART since 2007, presented with complaints of gradually progressive weakness and sensory loss in both lower limbs (right > left). There was no bladder or bowel involvement. His neurological system examination revealed features of compressive myelopathy due to a cauda equina syndrome. The rest of the general physical and systemic examination was normal. X-Ray of the sacral spine showed multiple lytic lesions [Table/Fig-3]. The MRI of the spine revealed a large, enhancing, lobulated, soft tissue mass lesion involving the sacrum extending into the spinal canal with another similar lesion involving L4 vertebral body and its posterior elements and a few small irregular lesions of the dorsal spine [Table/Fig-4]. There was an associated lytic, expansile, lesion with destruction of the cortex in the sacrum, obliterating the spinal cord. Lytic lesions were also noted involving the body of L4 vertebra on left side and on right 6th rib. A biopsy taken from the sacral mass [Table/Fig-4] consisted of sheets of cells that were CD138 positive. The serum electrophoresis was positive for an M spike in gamma globulin region. Bone marrow aspiration and biopsy showed increase in plasma cells to 9% of marrow nucleated cells, and there was no evidence of granulomas or lymphoma. A final diagnosis of plasmacytoma was made. The patient was started on chemotherapy with Vincristine/Adriamycin/Dexamethasone (VAD).
X-Ray of the sacral spine showing multiple lytic lesions. (Arrow show-ing the lytic lesions)
MRI spine revealing large enhancing lobulated soft tissue mass lesion involving the sacrum extending into the spinal canal with similar lesion involving L4 vertebral body. (Arrow highlighting the sacral mass) (Images from left to right).
Case 3
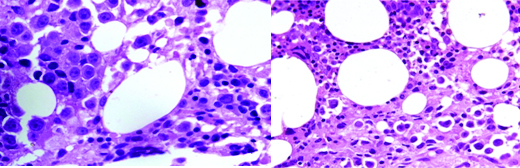
A 40-year-old male with HIV 1 infection on first line ART for three years, was being treated for multi drug resistant pulmonary tuberculosis for 18 months. He presented with complaints of breathlessness, cough and chest pain of seven days duration. On examination, he had a BP of 84/60 mmHg, pulse rate 92/minute, was pale and had a raised jugular venous pressure. Cardiovascular examination revealed muffled heart sounds. There was no other abnormality on general physical and systemic examination. An echocardiography showed a large pericardial effusion with cardiac tamponade. An emergent pericardiocentesis with pig-tail catheter insertion was done. Pericardial fluid analysis showed total count: 300 cells, differential count:polymorphs 80, lymphocytes 20, sugar: 133 mg/dl, protein: 3.9 gm/dl and ADA: 4.9 IU. On blood investigations, Hb was 8.5 gm/dl and peripheral blood smear revealed atypical cells: 3%, myelocytes: 4% and metamyelocytes: 3%. Rest of the investigations was normal. Despite being on ATT and steroids, the patient continued to have drainage of about 600 ml of pericardial fluid every day. Pericardial fluid for malignant cells was negative and serum Thyroid Stimulating Hormone (TSH) was normal. The TB-PCR of the fluid was also negative. A chest tomography revealed necrotic mediastinal lymph nodes with gross pericardial and bilateral pleural effusions. On the 20th day of admission, the patient reported severe back ache and examination revealed tenderness in the lumbosacral spine. The X-ray of lumbosacral spine revealed sclerosis of L5 and S1 with collapse and a lytic lesion in L4 vertebra. Bone marrow examination revealed paucity of myeloid precursors [Table/Fig-5]. The marrow was infiltrated by large, atypical cells with central to eccentric nuclei having 1-4 prominent nucleoli. Some of the cells showed perinuclear horf and cytoplasmic vacuolization. Immunohistochemical staining was diagnostic of plasmablastic lymphoma (CD138, CD 38 positive, CD 20 negative). The patient developed severe urosepsis and sepsis syndrome during the course of hospital stay and succumbed to his illness.
Bone marrow biopsy revealing paucity of myeloid precursors and the marrow is infiltrated by large atypical cells with central to eccentric nuclei having 1-4 prominent nucleoli. Some of the cells show perinuclear horf and cytoplasmic vacu-olization
Discussion
PLHIV are at an increased risk for plasma cell disorders that range from polyclonal hypergammaglobulinemia to aggressive Multiple Myeloma (MM), Monoclonal Gammopathy of Unknown Significance (MGUS), solitary plasmacytoma and less commonly plasmablastic myelomas and lymphomas [1]. Both transient and persistent paraproteinemia may be seen in these patients, which may not be due to a plasma cell dyscrasia. Antigen stimulation and Immunodeficiency are the important mechanisms proposed to lead to plasma cell disorders in PLHIV [2]. HIV antigens and antigens of other viruses like EBV, HHV 8 have been suggested to act as superantigens and stimulate B cell proliferation. T cell depletion and dysfunction due to HIV infection is directly implicated in the increased incidence of MM in PLHIV.
Among PLHIV, the incidence of monoclonal gammopathy and MM is higher than that of the general population and occurs at an earlier age [3-5]. The incidence of monoclonal gammopathy has been reported from 3.8% to 26% among PLHIV [6]. Similarly, for HIV infected patients with MM, the mean age in a series of 35 patients was 42 years [7].
According to Briault S et al., the predominant light chain in HIV related plasma dyscrasias was monoclonal immunoglobulin of lambda (λ) type without any monoclonal IgA immunoglobulin in their series of patients. They also displayed a much higher frequency of IgG3 and IgG4 M-proteins, and a much lower frequency of IgG1 M-proteins [8]. These patients also tend to have overall lower level of M-proteins.
MM in PLHIV shows atypical clinical patterns; it tends to present as solitary bone plasmacytoma or extramedullary plasmacytoma especially of the oral cavity. Plasma cell leukemia may also be one of the presentations. The disease progression is rapid and the prognosis is poor in many instances. In our cases, there were variable manifestations of plasma cell proliferation viz. plasmacytoma, plasmablastic myeloma and lymphoma.
Plasmacytic myeloma has varied cytologic features from small, lymphoplasmacytic cells to large plasmablasts with prominent central nucleoli and immature cytoplasm. Under the criteria of Greipp PR et al., [9], plasmablastic myeloma cells are defined as having a large, centrally placed nucleus (>10 μm in diameter) or a nucleolus (>2 μm in diameter), a high nuclear/cytoplasmic ratio, scant cytoplasm and absent prominent perinuclear horf. Plasmablasts must comprise > 2% of nucleated cells in the bone marrow aspirate to constitute Plasmablastic PCM. Bartl R et al., have suggested that for plasmablastic PCM, plasmablasts must be “the predominant cell type in the bone marrow biopsy specimen” [10].
Plasmablastic lymphoma comprise approximately 2% of all lymphomas in the setting of HIV infection [11]. Plasmablastic lymphoma has been described with a propensity to involve the oral cavity of HIV infected individuals. The features of plasmablastic lymphoma overlap with myeloma and quite often pose a diagnostic challenge. This is compounded by its aggressive, relapsing clinical course, high rates of disease progression and mortality [12]. Dong HY et al., reported that the median age of plasmablastic lymphoma patients was 38 years, male:female ratio was 7:1, and median CD4 count was 178 cells/mm3. Plasmablastic lymphoma are generally CD45 +, CD20-, PAX-5-, and CD138+. The most commonly involved location was the oral cavity (6 of 13 cases), followed by bone and soft tissue (4 of 13), and the gastrointestinal tract (3 of 13) [13].
For PLHIV with polyclonal hypergammaglobulinemia and monoclonal gammopathy, the current management strategy includes close follow up and ART. For symptomatic MM in these patients, chemotherapy with ART is recommended. The chemotherapy regimen in HIV infected patients is the same as that for the general population. Use of novel therapeutic agents followed by autologous stem cell transplantation has improved the outcome in some MM patients [2].
Overall, the incidence of plasma cell disorders appears to have increased in HIV positive patients owing to their longer survival with ART and management of opportunistic infections. Also, immunodeficiency itself is proposed to contribute to the increased incidence of plasma cell disorders. Their clinical presentations may be very varied: pancytopenia, medullary and extramedullary plasmacytomas, multiple myeloma or the more aggressive plasmablastic myelomas and lymphomas [14]. Early diagnosis and appropriate institution of chemotherapy is essential for managing these challenging cases.
Conclusion
To conclude, plasma cell dyscrasias are more frequent among people with HIV infections and have a very varied clinicopathological spectrum. The disease tends to be aggressive with more adverse outcomes among these patients. Awareness about these disorders among the physicians and care providers is needed for early diagnosis and management.
[1]. Dezube BJ, Aboulafia DM, Pantanowitz L, Plasma cell disorders in HIV-infected patients: From benign gammopathy to multiple myelomaAIDS Read 2004 14(7):372-74.377-79 [Google Scholar]
[2]. Coker WJ, Jeter A, Schade H, Kang Y, Plasma cell disorders in HIV infected patients: Epidemiology and molecular mechanismsBiomarker Res 2013 1:8 [Google Scholar]
[3]. Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Prevalence of monoclonal gammopathy of undetermined significanceN Engl J Med 2006 354(13):1362-69. [Google Scholar]
[4]. Frisch M, Biggar RJ, Engels EA, Goedert JJ, Association of cancer with AIDS-related immunosuppression in adultsJAMA 2001 285(13):1736-45. [Google Scholar]
[5]. Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Trends in cancer risk among people with AIDS in the United States 1980–2002AIDS 2006 20(12):1645-54. [Google Scholar]
[6]. Lefrere JJ, Debbia M, Lambin P, Prospective follow up of monoclonal gammopathies in HIV infected individualsBr J Haematol 1993 84(1):151-55. [Google Scholar]
[7]. Blade J, Kyle RA, Multiple myeloma in young patients: Clinical presentation and treatment approachLeuk Lymphoma 1998 30(5–6):493-501. [Google Scholar]
[8]. Briault S, Courtois-Capella M, Duarte F, Aucouturier P, Preud’ Homme JL, Isotypy of serum monoclonal immunoglobulins in human immunodeficiency virus-infected adultsClinExp Immunol 1988 74(2):182-84. [Google Scholar]
[9]. Greipp PR, Raymond NM, Kyle RA, O’Fallon WM, Multiple myeloma: Significance of plasmablastic subtype in morphological classificationBlood 1985 65(2):305-10. [Google Scholar]
[10]. Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W, Histologic classification and staging of multiple myeloma: A retrospective and prospective study of 674 casesAm J ClinPathol 1987 87:342-55. [Google Scholar]
[11]. Carbone A, AIDS-related non-Hodgkin’s lymphomas: From pathology and molecular pathogenesis to treatmentHum Pathol 2002 33(4):392-404. [Google Scholar]
[12]. Bibas M, Castillo JJ, Current knowledge on HIV associated plasmablastic lymphomaMediterr J Hematol Infect Dis 2014 6(1):e2014064 [Google Scholar]
[13]. Dong HY, Scadden DT, de Leval L, Tang Z, Isaacson PG, Harris NL, Plasmablastic lymphoma in HIV positive patients: An aggressive epstein-barr virus-associated extramedullary plasmacytic neoplasmAm J SurgPathol 2005 29(12):1633-41. [Google Scholar]
[14]. Gupta AP, Kumbhalkar DT, Parate SN, Kawthalkar SM, Nayak S, Kodate PM, HIV seropositivity and neoplasms with plasma cell morphology: Plasmablastic lymphoma and plasma cell myeloma: Is it a chance association or an increasing occurrence?Ann Pathol and Lab Med 2015 2(02):C111-17. [Google Scholar]