Invasive Cystic Hypersecretory Carcinoma of Breast: A Rare and Under Diagnosed Variant of Ductal Carcinoma
Nibedita Sahoo1, Pritinanda Mishra2, Susama Patra3, Prakash Kumar Sasmal4
1 Senior Resident, Department of Pathology, AIIMS, Bhubaneswar, Odisha, India.
2 Associate Professor, Department of Pathology, AIIMS, Bhubaneswar, Odisha, India.
3 Professor, Department of Pathology, AIIMS, Bhubaneswar, Odisha, India.
4 Assistant Professor, Department of Surgery, AIIMS, Bhubaneswar, Odisha, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Susama Patra, Professor, Department of Pathology, AIIMS, Bhubaneswar-761019, Odisha, India.
E-mail: wususama@gmail.com
Handful cases of invasive Cystic Hypersecretory Ductal Carcinoma (CHC) have been reported so far in literature. Cystic hypersecretory lesions of breast have a spectrum of morphological features ranging from Cystic Hypersecretory Hyperplasia (CHH), CHH with atypia, in situ to invasive CHC. We are reporting a case of a 32-year-old female who had nipple discharge and lump in her right breast for one month. A modified radical mastectomy was done and morphological diagnosis of invasive CHC with axillary node metastasis was made. The postoperative events were uneventful. Invasive CHC being a rare entity, our understanding of its biological behavior, prognostic factors and genetic basis is limited. The authors are aware of only 15 similar cases being reported in the English literature.
Breast carcinoma, Discharge, Lump, Mastectomy
Case Report
A 32-year-old female presented with serosanguinous nipple discharge and palpable mass in her right breast for one month. On examination the lump was located in the upper outer quadrant, of size 7x5 cm and was non tender. Fine needle aspiration of the breast lump was done and the aspirate was blood mixed mucoid. Microscopic examination of the smears revealed tumour cells in papillary pattern and cell balls in a mucoid like background, based on which a diagnosis of colloid carcinoma was given [Table/Fig-1a]. The patient had no history of benign breast disease previously or any family history of breast cancer. The patient underwent modified radical mastectomy.
a) Aspiration cytology showed tumour cells in papillary pattern and cell balls in a mucoid like background (H&E 40X); b) Mastectomy specimen showing multiple cysts with intervening greyish white areas; c) Cysts of various sizes filled with gelatinous mucoid material; d) Intraductal component with cystic dilatation and micropapillary pattern (H&E10X).
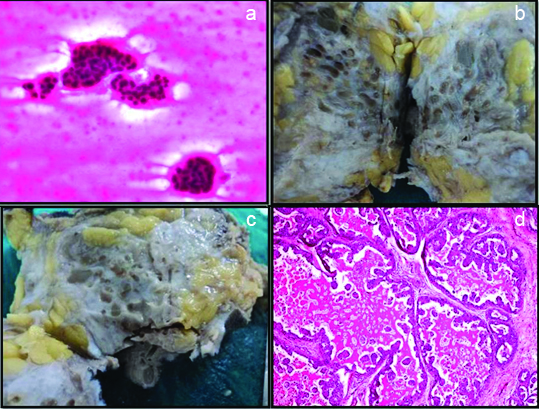
Grossly, the cut surface revealed a mass of size 6x4 cm in the central quadrant with characteristic presence of multiple cysts approximately 40 in number with a diameter varying from 0.2 to 1.5 cm, filled with thick grey to green colored gelatinous material [Table/Fig-1b and 1c]. Intervening areas were solid and grey white. The tumour was 0.5 cm away from the nipple areola complex and deep resected margin grossly appeared to be involved by the tumour. Other surgical margins were free of tumour involvement.
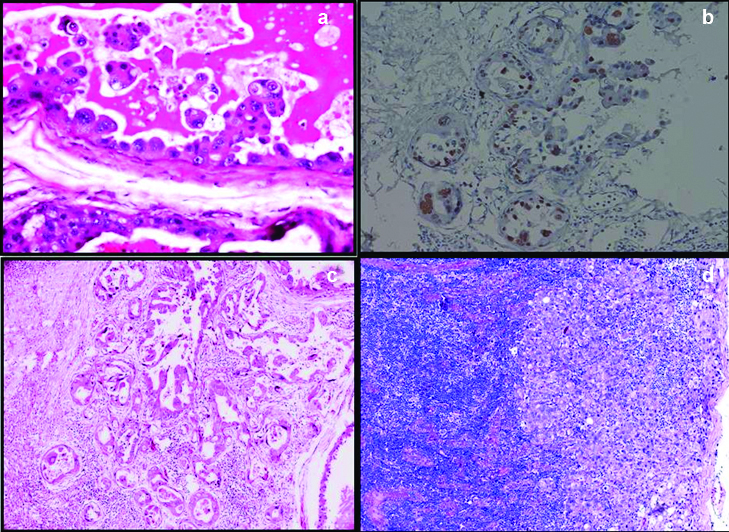
Histopathology of the tumour showed extensive intraductal component with cystically dilated ducts containing eosinophilic hyaline secretions which was diastase resistant PAS positive. At places the secretions were retracted from the surrounding epithelium producing a scalloped margin. The intraductal components showed a spectrum of epithelial patterns like micropapillary, cribriform, comedo and flat clinging type. Of all these findings micro papillary was the predominant pattern seen in our case [Table/Fig-1d]. The nuclear grade was intermediate to high grade [Table/Fig-2a]. Tumour cells showed patchy nuclear positivity for p53 [Table/Fig-2b]. The tumour infiltrated the stroma in nests and discretely with an associated inflammatory response [Table/Fig-2c]. There were lymphovascular invasion and two axillary lymph nodes showed macro metastasis [Table/Fig-2d]. Few areas showed cysts lined by flattened epithelium and was devoid of nuclear atypia. The nipple areola complex was free of tumour involvement and the deep resection margin was 0.2 cm from the tumour microscopically. Adjacent breast showed a tiny fibroadenoma.
a) Scalloping of secretions and tumour cells showing high nuclear grade (H&E 40X); b) Patchy nuclear positivity of p53 in the tumour cells. (IHC 20X); c) Tumour invasion in nests with stromal inflammatory response (H&E 40X); d) Lymph node showing macro metastasis (H&E, 10X).
Prognostic markers were ER negative, PR negative and HER2/neu 2+. FISH examination was negative for HER2/neu gene amplification. The postoperative course was uneventful, patient is with no evidence of disease for 28 months after surgery.
Discussion
CHC is an uncommon subtype of duct carcinoma, was first described by Rosen PP and Scott M in 1984 [1]. It is a distinctive variant of duct carcinoma. The gross and the microscopic features of this entity are different from the usual infiltrating duct carcinoma of no special subtype (IDC-NST). Gross examination shows numerous cysts of varying sizes containing gelatinous material and microscopically dilated ducts containing eosinophilic secretions. CHH is differentiated from CHC by absence of micropapillary pattern and cytological atypia in the lining epithelium. Most of the cases reported in the literature are in situ carcinoma and only 20% of cases have an invasive component [2,3]. This unusual variant of breast carcinoma which is not included in the latest WHO classification of breast cancer 2012, with a distinctive gross and microscopic features triggered us to report this rare entity. In the English literature only fifteen cases of invasive CHC have been reported so far [4]. Our case is the sixteenth case of CHC and fifth case of CHC with axillary node metastasis, which is still a rare finding.
The usual clinical presentation of CHC is a palpable lump with localised pain and rarely nipple discharge [5]. Our case presented with palpable mass and serosanguinous nipple discharge, which highlights the fact that presentation can be variable. D’Alfonso TM et al studied 10 cases of CHC, out of which 10% cases presented with bloody discharge [6]. Initially the patient was subjected for FNAC, where the cells were predominantly in small tight clusters, in a pool of mucin. Few areas also showed sheets of histiocytes embedded in a colloid like background.
There is very little literature regarding the cytological aspect of CHC. Lee WY et al described the distinctive cytological features of CHC and correlated with histology [7]. They described epithelial cells, showing a variety of cellular patterns, which were indistinguishable from usual ductal carcinoma cells in orange to grey-green thyroid colloid like background.
Extravasation of cyst material into the stroma is not considered as invasion [1,8,9]. The invasive foci shows solid nests, and is usually poorly differentiated with no hypersecretory characteristics [2,3]. Our case predominantly showed in situ carcinoma and invasion was in the form of small clusters and single cells, which indicated early invasion. Subsequent regrossing and sectioning revealed similar features in few of the sections which signifies extensive sampling should be done in CHC to look for the invasive component. Among the reported cases of cystic hypersecretory breast lesions, countable number of cases are invasive and rest are in situ. However the case concerned showed spectrum of lesions comprising of CHH, CHC in situ and foci showing invasion along with lymph node metastasis.
Though the differential diagnosis of the above case includes mucinous carcinoma, malignant mucocele-like tumour, juvenile secretory carcinoma and apocrine carcinoma. However, cystic hypersecretory carcinoma have pathognomonic gross and microscopic features which differentiates this entity from the above mentioned tumours.
Most invasive CHCs have been poorly differentiated duct carcinomas with a solid growth pattern unlike the present case. A total of four cases were diagnosed with lymph node metastasis. And one patient with invasive CHC, developed invasive lobular carcinoma of the contralateral breast 10 years after mastectomy [2]. But the question that needs to be answered at this point of time is that, is it different from usual invasive ductal carcinoma, if so then in what way? Is the prognosis better from the conventional ductal carcinoma? Should we report this entity or just mention in the reports that it is a variant of ductal carcinoma and the biological behavior of the tumour is comparable with usual carcinoma. Probably the answer to these questions can be clarified only by studying a larger series of the above entity.
Conclusion
Owing to less number of reported cases, little is known about the biological behavior, prognosis and molecular studies of this rare tumour. Therefore a larger series with long term follow up is required to unfold the biological behavior of this uncommon tumour.
[1]. Rosen PP, Scott M, Cystic hypersecretory duct carcinoma of the breastAm J Surg Pathol 1984 8(1):31-41. [Google Scholar]
[2]. Herrmann ME, Mc Clatchey KD, Siziopikou KP, Invasive cystic hypersecretory ductal carcinoma of breast: a case report and review of the literatureArch Pathol Lab Med 1999 123:1108-10. [Google Scholar]
[3]. Skalova A, Ryska A, Kajo K, Di Palma S, Kinkor Z, Michal M, Cystic hypersecretory carcinoma: rare and poorly recognized variant of intraductal carcinoma of the breast. Report of five casesHistopathology 2005 46(1):43-49. [Google Scholar]
[4]. Gupta P, Dhingra S, Musa O, Srivastava AN, lnvasive cystic hypersecretory carcinoma of the breast associated with papillary pattern: a rare and poorly recognised variant of ductal carcinoma of the breastecancer 2014 8:477 [Google Scholar]
[5]. Song SW, Whang IY, Chang ED, Cystic hypersecretory ductal carcinoma of the breast: a rare cause of cystic breast massJpn J Radiol 2011 29(9):660-62. [Google Scholar]
[6]. D’Alfonso TM, Ginter PS, Liu YF, Shin SJ, Cystic hypersecretory (in situ) carcinoma of the breast: a clinicopathologic and immunohistochemical characterization of 10 cases with clinical follow-upAm J Surg Pathol 2014 38(1):45-53. [Google Scholar]
[7]. Lee WY, Cheng L, Chang TW, Diagnosing invasive cystic hypersecretory duct carcinoma of the breast with fine needle aspiration cytology. A case reportActa Cytol 1999 43(2):273-76. [Google Scholar]
[8]. Guerry P, Erlandson RA, Rosen PP, Cystic hypersecretory hyperplasia and cystic hypersecretory duct carcinoma of the breastCancer 1988 61:1611-20. [Google Scholar]
[9]. Rosen PP, Cystic Hypersecretory Carcinoma and Cystic Hypersecretory HyperplasiaRosen’s Breast PathologyThird edLippincott Williams and Wilkins:581-89. [Google Scholar]