Pulmonary actinomycosis is a rare bacterial lung infection which is caused mainly by Actinomyces israelii. This non contagious infection can destroy parts of the lungs. There are variable presentations of pulmonary actinomycosis with similarity in manifestations to other infectious diseases of the lungs. Pulmonary actinomycosis is diagnosed by fine needle aspiration, bronchoscopy and finding of typical sulfur granules. We present a case of pulmonary actinomycosis in a middle aged (AIDS/HCV) man with massive hemoptysis and progressive dyspnoea. The bronchoscopy findings showed endobronchial mass with luminal occlusion in right upper lobe. Because of massive hemoptysis and poor response to conservative treatment and penicillin therapy, right upper lobectomy was needed to stop the bleeding. Histopathologic examination revealed the aggregations of filamentous Gram-positive organisms with characteristic pattern “sulfur granules”, indicating actinomycosis. The patient was followed by six months of oral amoxicillin and has no recurrent hemoptysis.
Hemoptysis, Immunodeficiency, Lung, Opportunistic infections
Case Report
A 52-year-old man with a history of AIDS/HCV since six years, was admitted to infectious department of Masih Daneshvari Hospital, Tehran, Iran, complaining of productive cough, progressive dyspnoea and massive hemoptysis (>550 ml each time). He also complained of gradual failure of breath, significant weight loss and anorexia since 20 days before visiting. He had a history of Deep Vein Thrombosis (DVT) six months ago and old-tuberculosis which had been treated nine months before current admission. Drugs given for tuberculosis: Isoniazid (INH), Rifampicin (RF), Pyrazinamide (PZ), and Ethambutol (ETB) for two months followed by INH and RF for seven months in a full compliance state. He was a smoker (30 pack-year) and addicted to heroin inhalation for the past 20 years, but in the last 12 years have been using an injection.
On admission, he was cachectic, unstable and afebrile. The patient’s blood pressure was 100/80 mmHg, pulse rate was 130 beats/min, respiratory rate was 33 breaths/min and oxygen saturation was 80% without nasal oxygen. He was slightly pale and his oral hygiene was poor. The cervical lymph nodes were not palpable. Oral candidiasis and clubbing of fingers due to long term smoking and Chronic Obstructive Pulmonary Disease (COPD) were found. Pulmonary auscultation revealed coarse crackles at the lung bases with tachypnoea and respiratory distress. Physical examination otherwise was normal. He often had taken combined oral spray of fluticasone 250 mcg and salmeterol 50 mcg for chronic bronchitis since four years.
The patient’s white blood count was 9900 cells/mm3 (lymphocytes, 2.1%, and neutrophils, 91.1%), hemoglobin was 8.1 gm/dL (normal range, 14-16 gm/dL), LDH was 675 U/L (normal range, 140-280 U/L), platelets were 265/μl (normal range, 150-450/μl) and ESR was 135 mm/hr (normal range, 0-29 mm/hr). The results of liver function tests and renal function tests were normal. Anti-HCV antibody was also reactive with patient’s serum and HCV-RNA was 200 million IU/ml detected by real-time PCR. Viral markers of HBS-Ag, HBc-Ab (IgM-IgG) and HBe-Ab were negative.
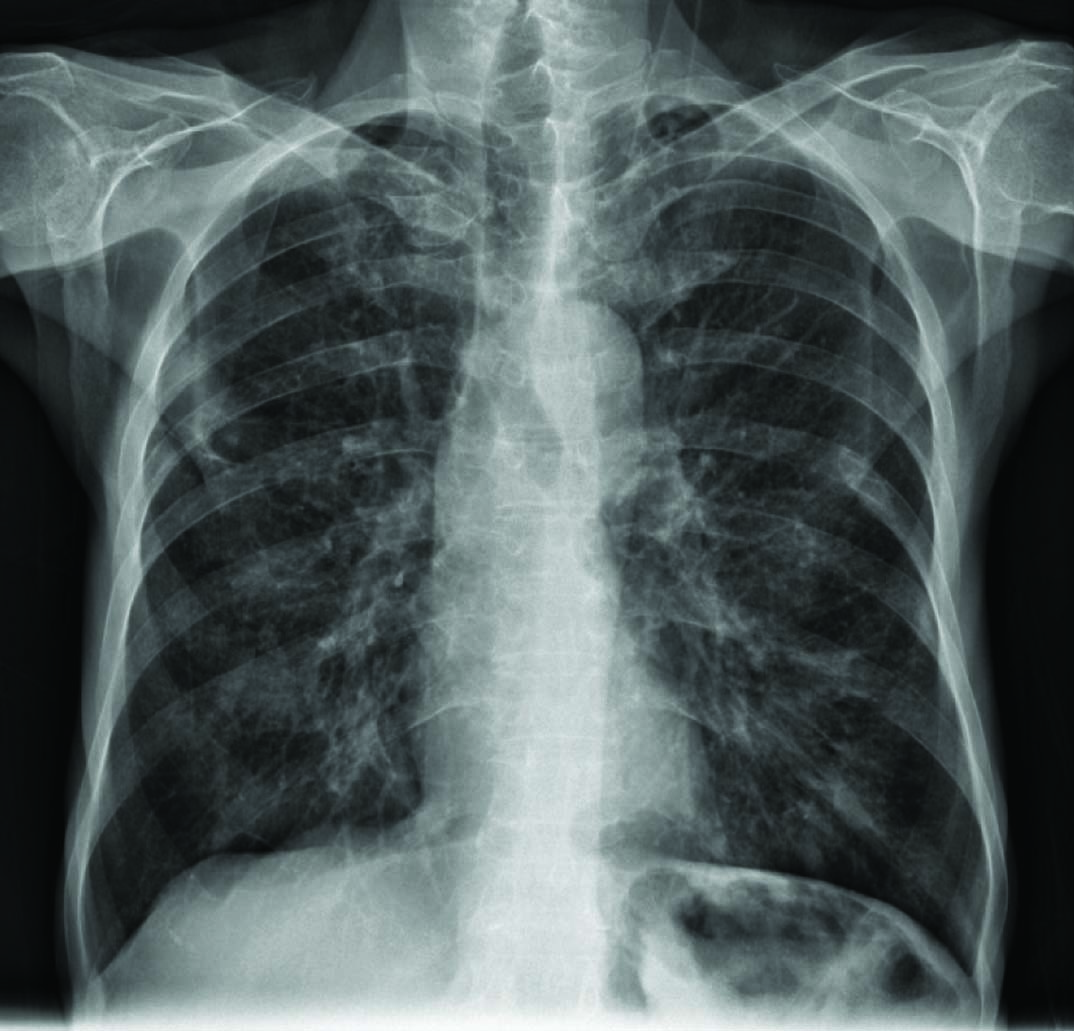
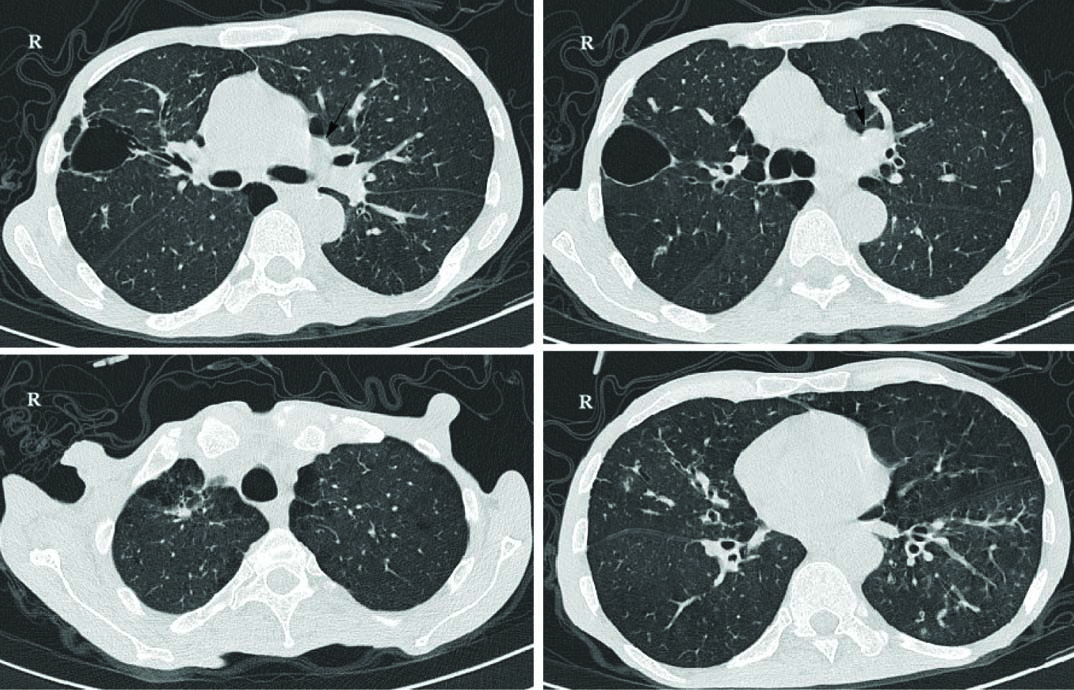
Two sputum smears for Acid-Fast Bacilli (AFB) were negative. Chest X-ray finding include interstitial infiltration, which might be owing to old tuberculosis [Table/Fig-1]. High Resolution Computed Tomography (HRCT) showed hyper-aerated lungs with evidence of peri-bronchial wall thickening and tubular bronchiectasis especially in mid zone and lower zone of fields [Table/Fig-2]. Centri-acinar emphysema and para-septal emphysematous bulla also were seen. Biapical parenchymal scar and fibrotic bands with apical pleural thickening were also reported which can be due to old TB changes. No evidence of active parenchymal infiltration was seen. There was a large mass like consolidation in left upper lobe with central necrosis and excavation and also adjacent nodular infiltration.
The Chest X-ray shows interstitial infiltration.
HRCT images show large mass like consolidation in left upper lobe with central necrosis and excavation and also adjacent nodular infiltration (black arrows, complete report of HRCT is in the text).
The bronchoscopy findings revealed endobronchial mass with luminal occlusion in right upper lobe. Microscopic findings showed numerous polymorphonuclear leucocytes and great number of microorganisms morphologically compatible with Actinomyces spp. The biopsy examination on Trans-Bronchial Lung Biopsy (TBLB) by bronchoscope revealed PAS positive large spherical cluster and branching “sulfur granules” which is specific for actinomycosis.
Conservative treatment was given including NPO, oxygen via nasal cannula at 3 l/min, and blood transfusion (2 units in 24 hours). A cardiovascular surgeon, thoracic surgeon and anesthesiologist were consulted. They suggested removing the involved tissues by surgery. Thoracotomy was performed after 36 hours of conservative treatment, under general anesthesia. It revealed a tense soft tissue mass in the right upper lobe with many collateral vessels. A right upper lobectomy was done. The final diagnosis of actinomycosis was established by detecting the actinomyces 16S rRNA gene using PCR in the removed lung tissues.
Seven days after surgery, there was no hemoptysis and the patient’s condition improved. Oral amoxicillin 1 gm eight hourly was started. After starting amoxicillin, the patient’s respiratory symptoms got better. Also clindamycin, metronidazole, trimethoprim/ sulfamethoxazole (TMP-SMX) and ceftriaxone were added to Highly Active Antiretroviral Therapy (HAART) (zidovudine, lamivudine 150 mg BD and efavirenz 600 mg daily) in addition to methadone for drug addiction was his discharge prescription. He was also suggested regarding pulmonary rehabilitation and was followed up for six months after discharge with no recurrence of hemoptysis.
Discussion
Actinomycosis is a granulomatous infection which were originally misclassified as fungi, but caused by anaerobic, non acid-fast Gram-positive bacterium [1]. Actinomycosis may be caused by six pathogenic species of Actinomyces but the most common species is Actinomyces israelii [2]. Herein, we presented a case of pulmonary actinomycosis in a 52-year-old man with HIV/HCV and history of COPD, DVT and treated pulmonary tuberculosis, which presented with hemoptysis and progressive dyspnoea.
Pulmonary actinomycosis is a rare infection with challenging issues for its diagnosis. This infection is presented in patients with poor oral hygiene and/or who are immunocompromised due to conditions such as uncontrolled diabetes [3], and AIDS [4,5], similar to the current case.
The affected lung contains granulomatous inflammation with purulent discharge containing yellowish sulfur granules [3]. The clinical signs of pulmonary actinomycosis may include weight loss, fatigue and low-grade fever and often may mimic the presentation of pulmonary infections or malignancies [6]. Most patients with pulmonary actinomycosis gradually develop a productive cough and chest pain. Hemoptysis is relatively uncommon, but life-threatening [6,7]. Our case also presented with chronic productive cough and massive hemoptysis, which magnified the importance of rapid diagnosis and management.
The incidence of pulmonary actinomycosis infection has decreased in last decades. This decline may be the result of better oral hygiene and also the administration of antibiotics in suspected lung infections [8]. In cases with high clinical suspicion of pulmonary actinomycosis, the diagnosis is commonly puzzled with other differentials such as suppurative lung diseases, pulmonary abscesses, and malignancies [7]. Studies have shown an association between chronic lung disorders e.g., COPD and bronchiectasis, with pulmonary actinomycosis [9]. The long-lasting history of smoking and heroin inhalation had led to chronic pulmonary disorders which made an appropriate background for catching uncommon pulmonary infections.
Radiologic studies of this case resulted in non specific discoveries which did not have the ability of differentiating possible diagnosis. The findings could be interpreted with the history of old treated TB and also lung cancer. The most common radiologic pattern of pulmonary actinomycosis is fibrotic infiltrates in single lobe with cavitary lesions but in advanced cases it penetrated through the chest wall and invaded bone tissue [10]. Radiologic findings are usually single or multiple masses in lungs with decreasing of the shadow in the center of these masses. For segregation of pulmonary actinomycosis and other differential diagnosis such as lung cancer “open bronchus” sign can be helpful because bronchus are obstructed or compressed in lung cancer [11]. The CT-scan findings include multifocal nodular looking, cavitation, air space consolidation, mass, pleural effusion and hilar lymphadenopathy [12].
Finally, our diagnosis was made based on TBLB as culture results were not beneficial. Bacterial culture is obtained in 50% of suspected actinomycosis patients because of the high percentage of false-negative results, due to difficulty in actinomyces culturing, previous antibiotic therapy and existence of other bacterial overgrowth. Examination of secretions of bronchus or sputum may give false-positive results in one third of patients because actynomyces can be a part of the normal flora of saliva [11].
Recommended treatment for pulmonary actinomycosis is antimicrobial therapy using beta-lactam antibiotics. The drug of choice is penicillin G. In addition, ampicillin, amoxicillin, clindamycin and roxithromycin can also be used [8,13]. In our case, owing to life-threatening massive hemoptysis and also consultation with cardiac and thoracic surgeons, the patient underwent lobectomy with following antibiotic therapy.
Conclusion
Pulmonary actinomycosis is a rare disease which has large diagnosis overlap with other lung disorders i.e., malignancies. It should be included in differential diagnosis especially in immunocompromised patients with chronic respiratory symptoms and hemoptysis.
[1]. Bose M, Ghosh R, Mukherjee K, Ghoshal L, Primary cutaneous actinomycosis: a case reportJ Clin Diagn Res 2014 8(7):YD03-05. [Google Scholar]
[2]. Wong VK, Turmezei TD, Weston VC, ActinomycosisBMJ 2011 343:d6099 [Google Scholar]
[3]. Mani RK, Mishra V, Singh PK, Pradhan D, Pulmonary actinomycosis: a clinical surprise!BMJ Case Rep 2017 :bcr2016218959 [Google Scholar]
[4]. Ghosh P, Gupta I, Kar M, Nandi P, Naskar P, Co-infection of Candida parapsilosis in a patient of pulmonary actinomycosis-a rare case reportJ Clin Diagn Res 2017 11(1):DD01-02. [Google Scholar]
[5]. Pierre I, Zarrouk V, Noussair L, Molina JM, Fantin B, Invasive actinomycosis: surrogate marker of a poor prognosis in immunocompromised patientsInt J Infect Dis 2014 29:74-79. [Google Scholar]
[6]. Skehan N, Naeem M, Reddy RV, Endobronchial actinomycosis: successful treatment with oral antibioticsBMJ Case Rep 2015 [Google Scholar]
[7]. Mabeza G, Macfarlane J, Pulmonary actinomycosisEuropean Respiratory Journal 2003 21(3):545-51. [Google Scholar]
[8]. Smith AJ, Hall V, Thakker B, Gemmell CG, Antimicrobial susceptibility testing of actinomyces species with 12 antimicrobial agentsJ Antimicrob Chemother 2005 56(2):407-09. [Google Scholar]
[9]. Tsubochi H, Endo S, Suhara K, Sohara Y, Endobronchial aspergillosis and actinomycosis associated with broncholithiasisEur J Cardiothorac Surg 2007 31(6):1144-46. [Google Scholar]
[10]. El Ghannam H, Bai C, Qiao R, Pulmonary actinomycosis presenting as a mass-like consolidationSouth Med J 2010 103(1):81-83. [Google Scholar]
[11]. De la Espina MA, Lopez-Menendez C, Ruiz-Martinez R, Molino-Trinidad C, Pulmonary actinomycosis with thoracic soft tissue mass: a rare onset formEur J Radiol 2001 37(3):195-99. [Google Scholar]
[12]. Costiniuk CT, Voduc N, de Souza C, Pulmonary actinomycosis in a male patient with a tracheal bronchusCan Respir J 2011 18(2):84-86. [Google Scholar]
[13]. Brook I, Actinomycosis: diagnosis and managementSouth Med J 2008 101(10):1019-23. [Google Scholar]