Local Injection of Triamcinolone Acetonide: A Forgotten Aetiology of Cushing’s Syndrome
Weera Sukhumthammarat1, Prapaipan Putthapiban2, Chutintorn Sriphrapradang3
1 Research Fellow, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
2 Research Fellow, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
3 Assistant Professor, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Chutintorn Sriphrapradang, 270 Rama 6 Road Ratchatewi, Bangkok, 10400, Thailand
E-mail: chutins@gmail.com
Many different non systemic corticosteroid administrations can cause iatrogenic Cushing’s Syndrome (CS). We herein report a case series of iatrogenic CS from keloid scars treatment and aesthetic regimen called mesotherapy. Our first patient developed CS after having exceeded recommended dose of intralesional injection of Triamcinolone Acetonide (TAC). Second case presented with CS followed by unidentified mesotherapy treatment for local fat reduction. Subcutaneous injections of dexamethasone were found to be the part of mesotherapy regimen in one case. Physicians should be insightful in prescribing TAC especially in those patients who have high predisposing factors for developing CS. In the same way, off-label mesotherapy combine with corticosteroid can lead to iatrogenic CS and Hypothalamic-Pituitary-Adrenal (HPA) axis suppression. Currently, there are no standard guidelines for mesotherapy treatment. Therefore, further clinical trials on dosage, duration and effective combination of mesotherapy regimens are needed to increase safety uses.
Adrenal insufficiency, Iatrogenic disease, Intralesional, Mesotherapy, Steroids
Case Report
Non systemic corticosteroid therypy is a preferred treatment modality to avoid systemic adverse effects. However, iatrogenic CS has been occasionally reported from many non systemic uses of corticosteroids e.g., topical and intralesional routes [1,2]. The spectrums of clinical presentation are broad and cannot be differentiated from endogenous CS [3].
Corticosteroids are widely used in dermatologic diseases. For example, intralesional corticosteroid is the first-line recommendation for treating keloid and hypertrophic scar [4]. Moreover, some of the off-label used treatments such as “mesotherapy” are adulterated with steroids to alleviate inflammatory effects of lipolytic agents. Standard treatment of dermatologic diseases or off-label aesthetic treatments that contain corticosteroids can lead to systemic adverse outcome like CS. We herein report three cases of systemic adverse effects following intralesional and subcutaneous corticosteroid administration.
Case-1
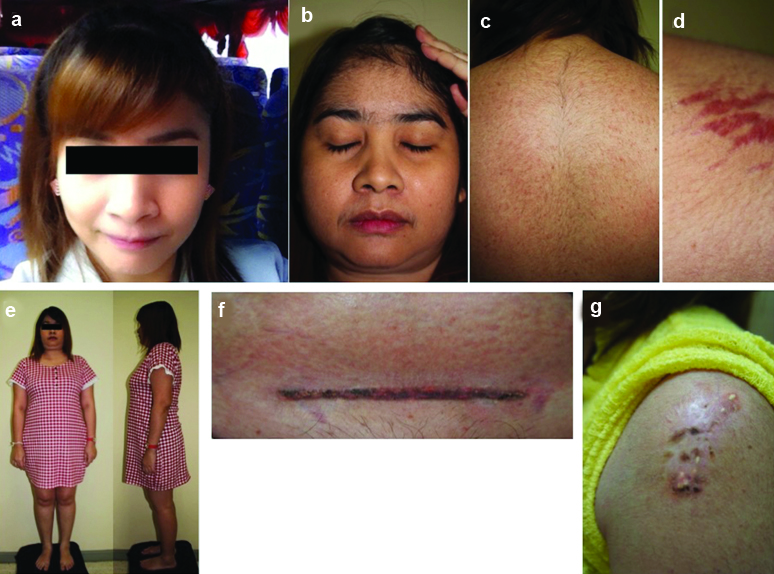
A 34-year-old female presented to a primary physician with increased facial hair for two months. She had secondary amenorrhea and weight gain of 10 kg within three months. On examination, she had a typical cushingoid appearance [Table/Fig-1]. Her blood pressure was 142/94 mmHg and her Body Mass Index (BMI) was 27.13 kg/m2. She denied history of oral corticosteroid use. With rapid onset of CS, adrenocortical carcinoma was initially suspected. Laboratory investigations showed morning serum cortisol <1 μg/ dl, undetectable 24-hour urinary free cortisol (3.5-45 μg), serum dehydroepiandrosterone-sulfate <15 μg/dl (35-430) and serum testosterone <20 ng/ml (20-81). Computed tomography of abdomen showed no adrenal or ovarian mass. After meticulous history review, she admitted that she had treated her keloid scars with intralesional TAC, 100 mg per month for six months before the visit. Keloid scars were observed at left shoulder and caesarean section incision [Table/Fig-1]. From medical records obtained from other hospital, it was observed that the treating physicians have exceeded the TAC recommended dosage. Her clinical appearance gradually improved when prednisolone was tapered down. Her HPA axis function resumed with morning serum cortisol of 17.2 μg/dl at 15.5 months.
Clinical features of case-1: (a) Self-photograph was obtained before the treatment of keloid scar with intralesional triamcinolone acetonide (TAC); (b) After TAC injection, there were several features of CS such as moon face, increase facial hair; (c) hirsutism; (d) purplish striae; (e) weight gain and truncal obesity. (f) keloid scars at caesarean section incision scar and (g) left shoulder after intralesional TAC injection.
Case-2
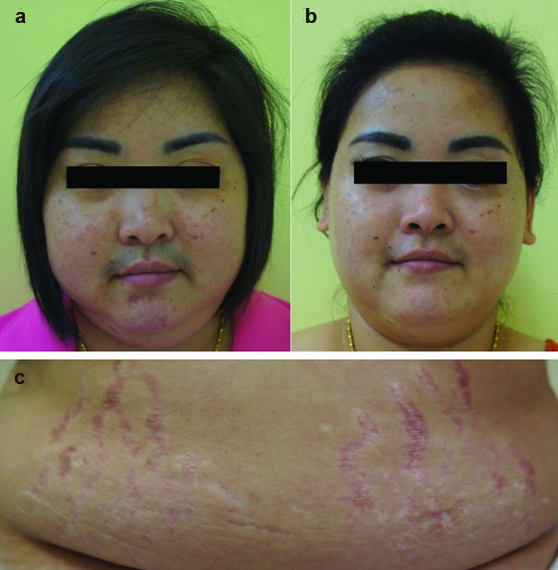
A 32-year-old female presented with unexplained weight loss from 130 kg to 110 kg in two months. She noticed an increase in facial hair and acne, swelling of face, and secondary amenorrhea [Table/Fig-2]. Four months prior, she had a treatment so-called “mesotherapy” for reducing fat. At an illegal clinic, she had subcutaneous injection with unidentified plant extract agents at both hips and arms every week for three months. The total dose was 50 vials (5 ml/vial). One week after completion, she had been gradually developing CS as described. Her blood pressure was 144/102 mmHg. Examination revealed moon face with hirsutism, erythematous striae at abdomen along with proximal muscle weakness. Endocrinological evaluation revealed suppressed cortisol (<1μg/dl). A diagnosis of exogenous CS was made. She was initially prescribed prednisolone 5 mg/ day with taper off. Five months later, her symptoms of CS were improved. Her HPA axis function recovered (morning serum cortisol of 14.3 μg/dl) at 11.5 months.
Clinical features of case-2, (a) before and (b) after cessation of “mesotherapy” treatment for five months, her swelling of face and facial hair decreased; (c) Wide purplish striae were presented on her abdomen.
Case-3
A 54-year-old female presented with complaints of increased facial swelling, hair loss, and fatigue for two months. Four months prior, she had a local fat reduction treatment called “mesotherapy”. The subcutaneous injection of unidentified agents plus 4 mg of dexamethasone were administered at her abdomen every week. Her total dose of dexamethasone was 36 mg in three months. On examination, her BMI was 20.34 kg/m2 and her blood pressure was 150/90 mmHg. She had a round face and fullness of supraclavicular fat pad. Hormonal evaluation confirmed suppressed morning cortisol <1 μg/dl and ACTH <5 pg/ml (0-46). A diagnosis of exogenous CS was concluded. After the diagnosis, she was prescribed prednisolone 5 mg/day with taper off. Four months later, her clinical signs and symptoms improved. Her HPA axis function gradually recovered at 3.5 months after her last mesotherapy.
Discussion
Intralesional TAC is the most widely used treatment for keloids and hypertrophic scars [5]. The recommended dose is TAC, 10 to 40 mg at six-week intervals [4]. Systemic side effects like CS may occur after frequent repeated injections leading to a high cumulative dose (>40 mg of TAC per month) [2]. Our first patient had received 100 mg of TAC every month for six months, which was a culprit cause of iatrogenic CS. One multinational survey revealed that 30% of plastic surgeons exceed the recommended dosage of TAC. Surprisingly, almost 50% are not aware of possible systemic adverse effects such as CS from intralesional TAC [2].
Besides standard treatment of skin diseases, non systemic corticosteroids are used in mesotherapy cocktail. Lipolytic stimulators and ablative mesotherapy are the two main mechanisms for local fat reduction. Infection and inflammatory skin complications are most commonly reported adverse outcomes of mesotherapy [6]. Although, there were unknown exact contents of the regimen of our second case, we can assume combination of corticosteroid from her clinical presentation and resolution of CS after cessation of mesotherapy. In our third case, total of 36 mg of dexamethasone was added to mesotherapy for three months. These two cases developed CS with laboratory proven HPA axis suppression. Despite widely aesthetic use of mesotherapy, there are no standard guidelines or FDA approval of mesotherapy as a cosmetic treatment.
The development of CS after a single and low dose of glucocorticoid has also been reported [7]. Other non systemic routes of corticosteroid usage such as topical and intralesional are also reported [1,2]. Not only with exceeded recommended dosage, CS was also found when approved dose or even a single dose of non-systemic corticosteroid was given [8]. In addition to dose and duration, drug interaction and physiologic hypercortisolism including obesity and poorly controlled diabetes mellitus are predisposing factors of iatrogenic CS [9,10]. Moreover, concealed adulteration of corticosteroid can be found and leads to difficulty of diagnosis.
Recovery of HPA axis after keloid treatment with intralesional TAC was varied and has been reported persisting up to two years [2]. One possible explanation contributing to long action of intralesional TAC is that keloid scars acting as a slow release reservoir [11].
Conclusion
Treatment with intralesional corticosteroid should be considered as serious as systemic corticosteroid therapy. Least possible dosage, early detection and appropriate follow up can be potential solutions for alleviating systemic complications. Equally important, further clinical studies on standardized treatment of mesotherpy are needed to increase safety concern. As a result of continuation off-label of mesotherpy, risks might overcome benefits.
Consent: Written informed consent was obtained from the patients for the publication of this case series and any accompanying images.
[1]. Decani S, Federighi V, Baruzzi E, Sardella A, Lodi G, Iatrogenic Cushing’s syndrome and topical steroid therapy: case series and review of the literatureJ Dermatolog Treat 2014 25(6):495-500. [Google Scholar]
[2]. Fredman R, Tenenhaus M, Cushing’s syndrome after intralesional triamcinolone acetonide: a systematic review of the literature and multinational surveyBurns 2013 39(4):549-57. [Google Scholar]
[3]. Sharma ST, Nieman LK, Feelders RA, Cushing’s syndrome: epidemiology and developments in disease managementClin Epidemiol 2015 7:281-93. [Google Scholar]
[4]. Juckett G, Hartman-Adams H, Management of keloids and hypertrophic scarsAm Fam Physician 2009 80(3):253-60. [Google Scholar]
[5]. Lumenta DB, Siepmann E, Kamolz LP, Internet-based survey on current practice for evaluation, prevention, and treatment of scars, hypertrophic scars, and keloidsWound Repair Regen 2014 22(4):483-91. [Google Scholar]
[6]. Jayasinghe S, Guillot T, Bissoon L, Greenway F, Mesotherapy for local fat reductionObes Rev 2013 14(10):780-91. [Google Scholar]
[7]. Motawea M, Mosaad H, Aladeeb NM, El-khalek AMA, Cushing syndrome following single steroid injection: a case report and review of the literatureAmerican Journal of Medical Case Reports 2016 4(4):130-33. [Google Scholar]
[8]. Iglesias P, Gonzalez J, Diez JJ, Acute and persistent iatrogenic Cushing’s syndrome after a single dose of triamcinolone acetonideJ Endocrinol Invest 2005 28(11):1019-23. [Google Scholar]
[9]. Nieman LK, Cushing’s syndrome: update on signs, symptoms and biochemical screeningEur J Endocrinol 2015 173(4):M33-38. [Google Scholar]
[10]. Schwarze-Zander C, Klingmuller D, Klumper J, Strassburg CP, Rockstroh JK, Triamcinolone and ritonavir leading to drug-induced Cushing syndrome and adrenal suppression: description of a new case and review of the literatureInfection 2013 41(6):1183-87. [Google Scholar]
[11]. Finken MJ, Mul D, Cushing’s syndrome and adrenal insufficiency after intradermal triamcinolone acetonide for keloid scarsEur J Pediatr 2010 169(9):1147-49. [Google Scholar]