Otomycosis or fungal otitis externa has been described as chronic superficial fungal infection of the External Auditory Canal (EAC). It is worldwide in distribution with prevalence ranging from 9% to 30% among patients presenting with signs and symptoms of otitis externa and discharging ears in otolaryngology settings [1-4]. The frequency of otomycosis depends upon different climatic conditions with higher prevalence in the hot, humid, and dusty areas of the tropics and subtropics [2-4]. Overview of the literature reveals otomycosis to be a common medical problem in India [5, 6].
Various local and systemic host factors have been proposed to be predisposing for otomycosis as instrumentation, relatively high humidity in EAC, accumulation of epithelial debris, long term use of broad spectrum antibiotics or steroid preparations, immuno-compromised status, co-morbid conditions like diabetes and dermatological diseases [7]. Patients suffering from otomycosis commonly present with pruritus, otalgia, aural fullness, tinnitus, hearing impairment, blocking sensation and ear discharge [4,7,8].
Despite the fact that climatic conditions in Haryana, India predispose the people to develop otomycosis, literature search reveals that not much work has been carried out in this region. The present study is designed with an objective to elucidate the predisposing factors, clinical presentations, mycological agents and associated bacteria encountered in clinically diagnosed otomycosis cases.
Materials and methods
A prospective study was conducted in the Department of Microbiology and Department of Ear, Nose and Throat, SGT hospital, located in a rural area of District Gurgaon, Haryana, India. A total of 350 patients, attending outpatient Department of Ear, Nose and Throat, were chosen for the study. The study was performed over eight month period from March to October 2016.
Ethical clearance was obtained from Institutional ethical committee. A predesigned proforma was used to evaluate and analyze demographic profile, predisposing factors, presenting complaints and clinical findings of clinically diagnosed patients.
Inclusion Criteria: 350 patients of more than five years of age with clinical diagnosis of otomycosis were included in the study. Criteria for establishing the diagnosis of otomycosis was based on the presence of symptoms like itching, pain, feeling of blocked ear, tinnitus, deafness, discharge and otoscopic findings revealing wet or dry matted masses of hyphae/spores or thick, white cheesy material [11,12].
Exclusion Criteria: All patients with history of chronic otitis media, tympanic membrane perforations, prior ear surgery or aural procedures were excluded. Diabetics and patients with chronic and serious debilitating illnesses like tuberculosis and malignancies were also excluded from the study.
Sputum smear examination for pulmonary tuberculosis and blood sugar estimation (level above 100 mg per deciliter excluded) were done to rule out these chronic diseases among study population.
Age, sex, socio-economic status, and occupation of the patients were recorded. Any history of use of wooden sticks, metal wax pickers or any other objects in an attempt to remove ear wax from ear, use of oil and topical antibiotic/steroid ear drops was noted. Clinical presentations of patients such as itching, pain, feeling of ear blockage, ear discharge and tinnitus were also recorded.
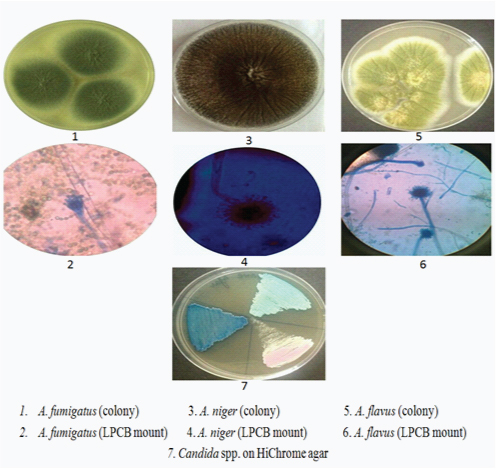
Sample Collection and Processing: EAC of each patient was examined in order to look for appearance of debris suggestive of fungal infection. Samples from EAC were collected with the help of sterile swabs or probe with curette and cotton carrier and transported to the laboratory within half an hour for mycological and bacteriological examination. Samples were collected from one ear only. All samples were evaluated by both direct examination and culture method. A portion of the sample was cultured on blood and MacConkey agar at 37°C for 48 hr. and examined for bacterial growth. Identification of the bacterial isolates was done by standard bacteriological procedures [11]. For mycological identification, direct microscopic examination was carried out by 10 % KOH examination and inoculation of material was done on two slants of Sabouraud’s Dextrose Agar (SDA) with chloramphenicol (Himedia, India), which was incubated at 25°C and 37°C aerobically for a period of 4 weeks. Culture media were examined for presence of colonies every 3-4 days [12]. Identification was done on the basis of colony morphology and Lactophenol Cotton Blue (LPCB) mount microscopy. Aspergillus isolates were chracaterized by varying length of conidiophores and extent of coverage of vesicles by phialides and conidia. For characterization of Candida isolates, germ tube test was done by observing the production of germ tubes on isolates in serum after 1-2 hours of incubation at 37°C and colonies were inoculated on HiChrome agar for identification of species [12].
Post collection all patients were subjected to a thorough aural toilet, following which patients were prescribed clotrimazole antifungal drops, 4-5 drops to be instilled once a day for 10-14 days. All patients were followed up after 14 days.
Statistical Analysis
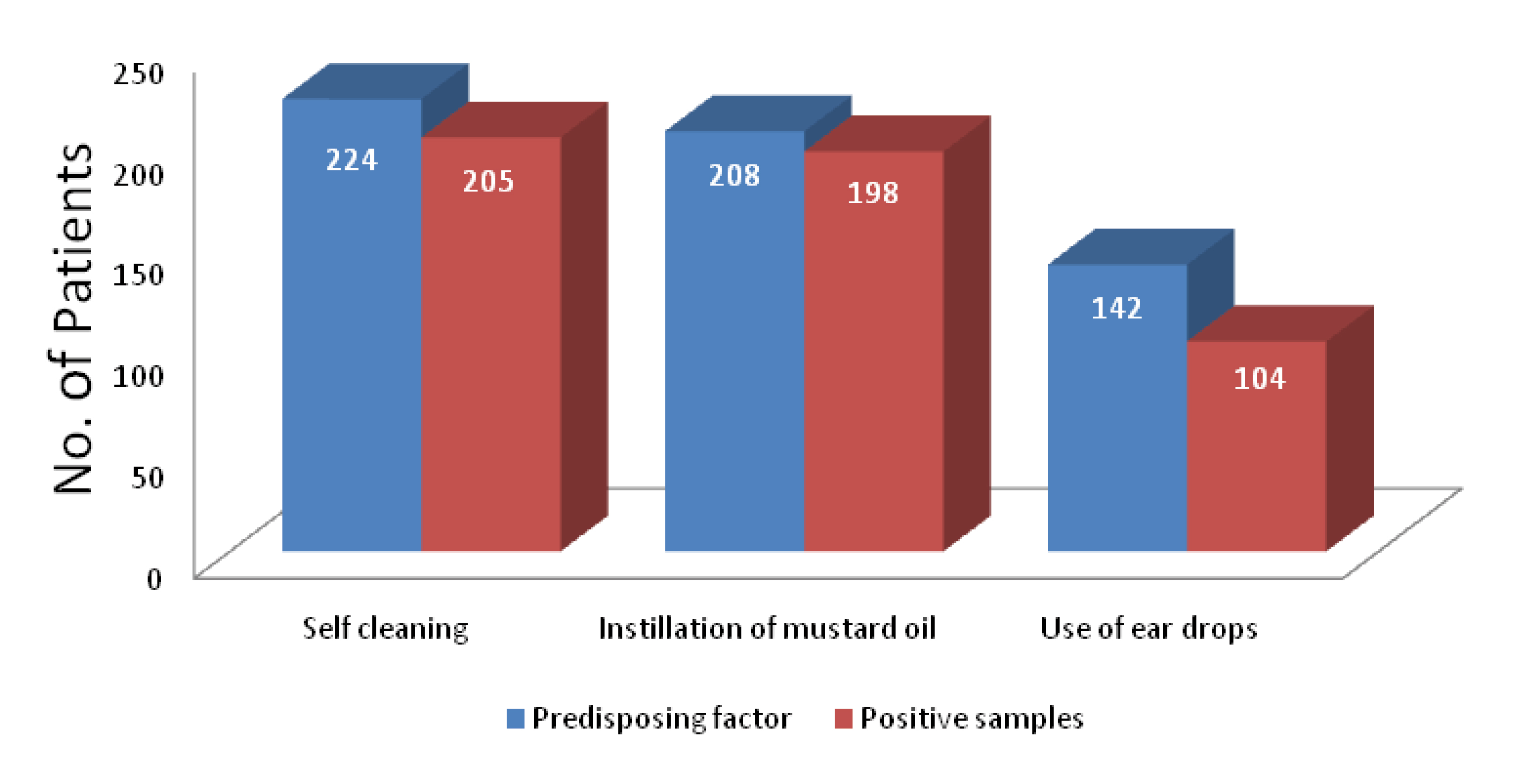
Categorical data was presented as frequencies and percentages. The association between the pre-disposing factors (self-cleaning, eardrops, and oil instillation) and the prevalence of otomycosis was analyzed with the chi-square test. p values below 0.05 were considered significant.
Results
Demographic profile of patients
Out of the total 350 patients, maximum number of patients belonged to the age group of >15-35 years followed by those in the >35-55 years age group. Among 350 study subjects, 199 were males and 151 females with male to female ratio as 1.3:1 [Table/Fig-1]. Occupation of majoirity of the males in the study population was farming (58%) and construction work (30%). Most (62%) of the females were housewives. Average monthly family income was below INR 8,000 in 57.1% patients.
Demographic profile of study subjects.
| Age | Study population (n= 350) |
|---|
| Male /Female Ratio | n (%) |
|---|
| 5- 15 years | 1.7:1 | 30 (8.6%) |
| >15- 35 years | 1.2:1 | 232 (66.3%) |
| >35- 55years | 1.4:1 | 80 (22.9%) |
| >55 years | 3: 1 | 8 (2.3%) |
Predisposing factors and Clinical features
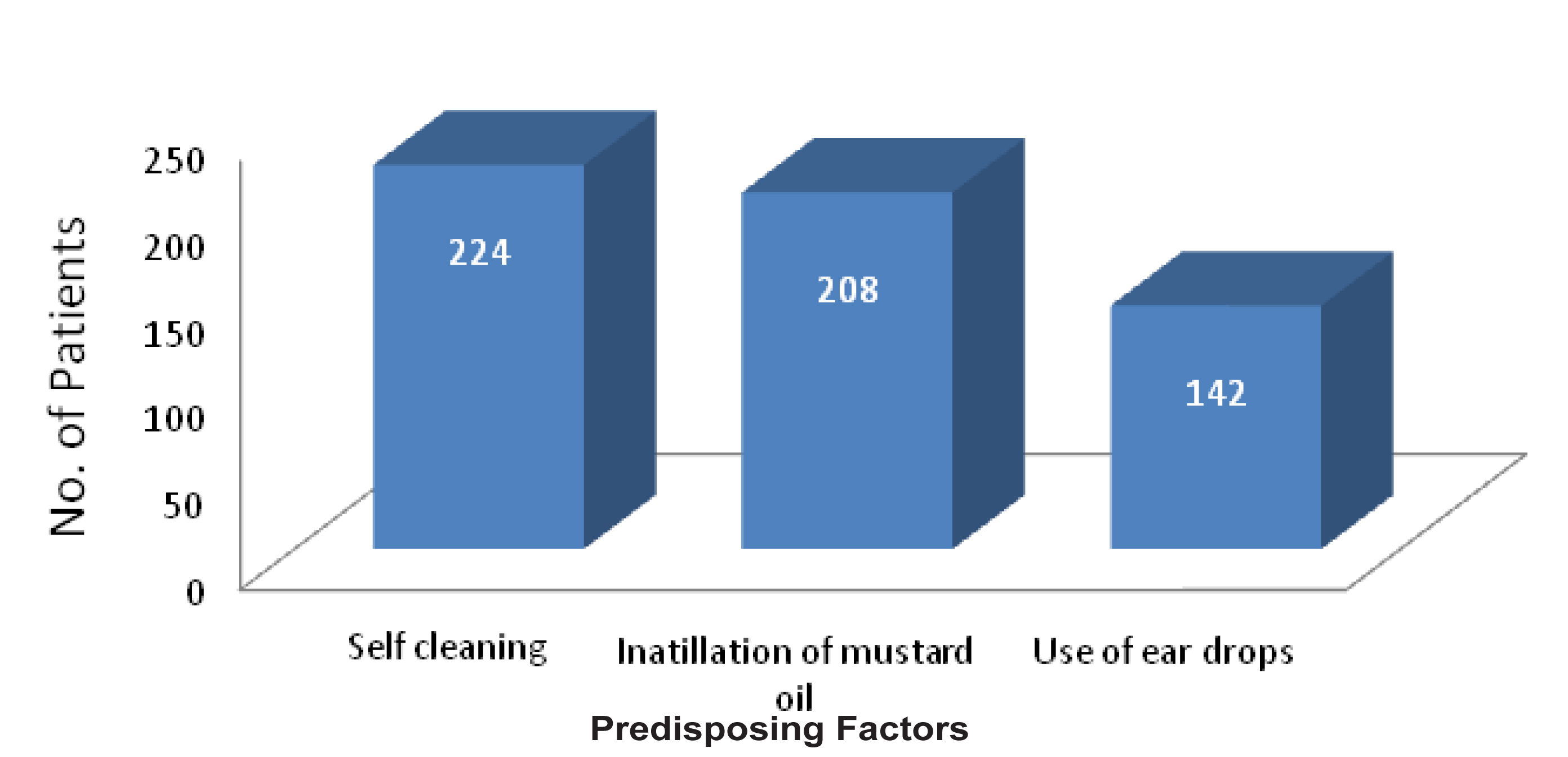
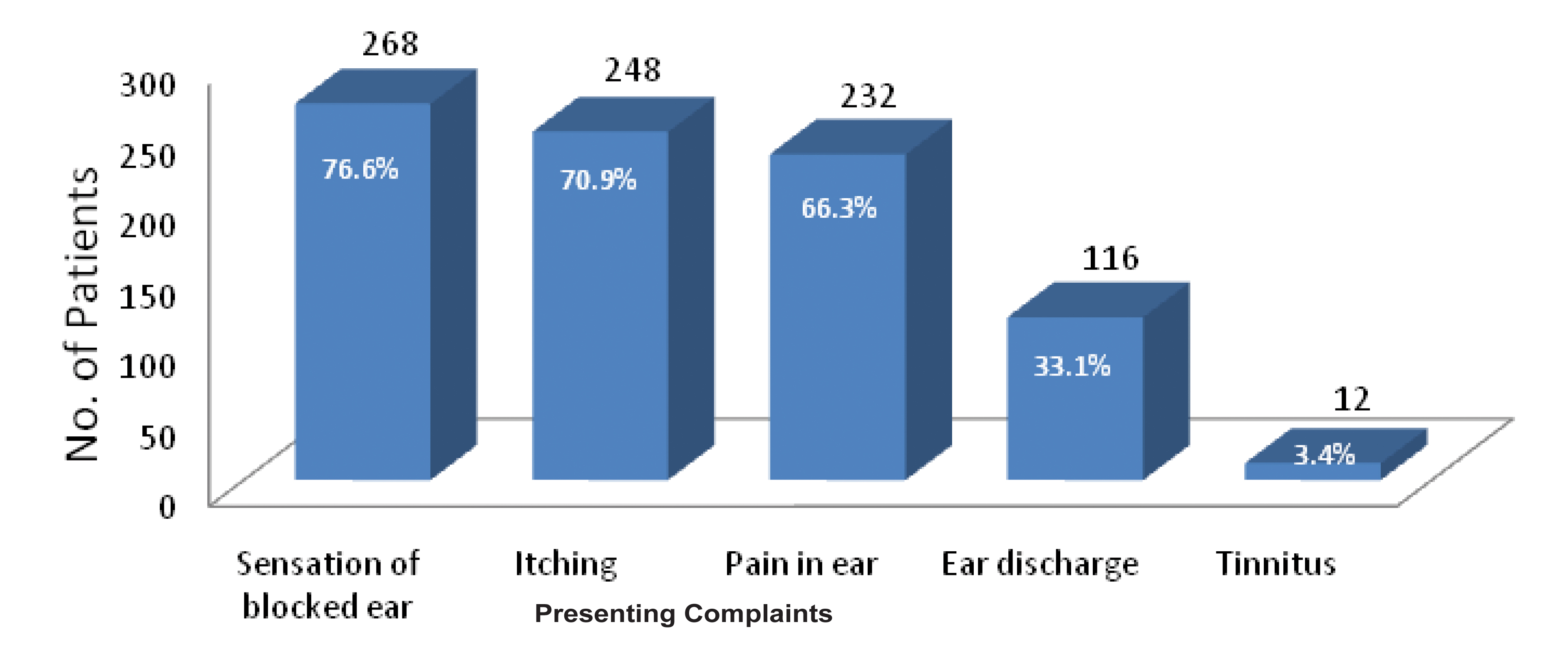
Involvement of one ear was observed in 96.6% patients, presented with suspected otomycosis. Right ear was involved in majority of the cases. Majority of the patients gave the history of frequent self cleaning of the ear with the help of unsterile pointed objects like match sticks and hair pins (in females). Practice of putting mustard oil and use of antibiotic ear drops were recorded in 59.4% and 40.6% patients respectively [Table/Fig-2]. Most common presenting complaint was blocking sensation of the ear, followed by itching, otalgia, ear discharge and tinnitus [Table/Fig-3].
Distribution of patients according to predisposing factors.
Distribution of patients according to presenting complaints.
Microbiological findings
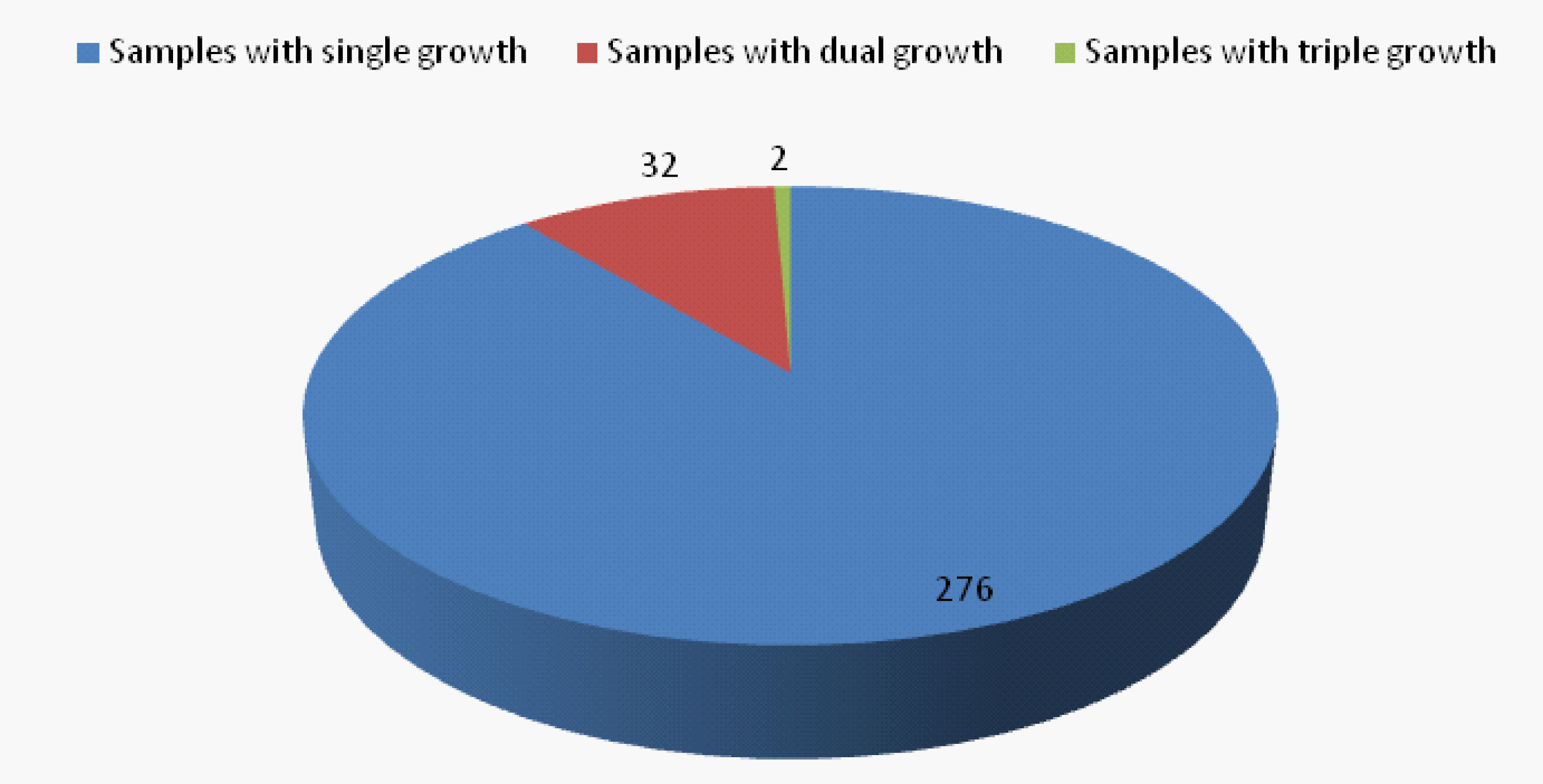
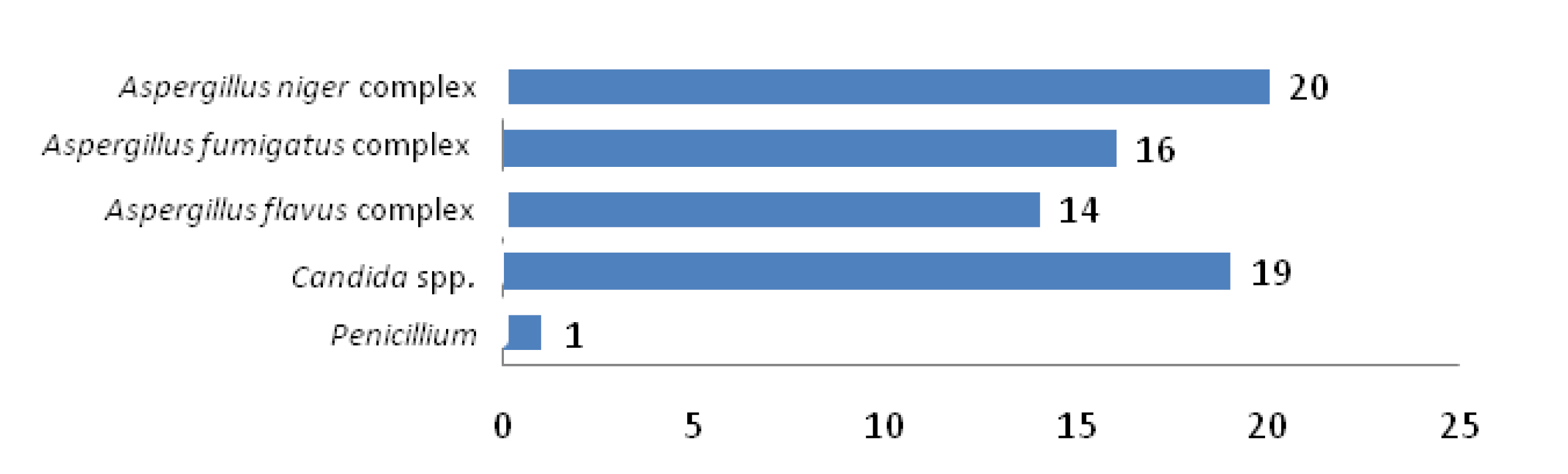
Fungal cultures yielded 346 fungal isolates in 310 samples from a total of 350 clinically diagnosed cases of otomycosis. Fungal culture positivity rate is 88.6%. Significant association was observed between various predisposing factors and positive samples [p<0.05] [Table/Fig-4]. Aspergillus spp. are the predominant fungi (n=302;87.3%) among total fungal isolates, more frequent being A. niger complex (n=206;68.2%) followed by A. flavus complex (n=60;19.9%) and A. fumigatus complex (n=36;11.9%). Other fungi isolated were Candida spp. (n=35;10%) Penicillium (n=5;1.4%), Mucor (n=2;0.58%) and Trichophyton mentagrophyte (n=2;0.58%). Associated bacterial infections were seen in 148(47.7%) cases. Commonest bacteria isolated were S. aureus (57.4%) followed by Pseudomonas aeruginosa (21.6%), Klebsiella spp. (16.9%), Proteus spp. (2.7%) and E. coli (1.4%) [Table/Fig-5]. Out of the total 310 positive samples, 32 samples yielded mixed growth with two fungi and two samples with three fungi [Table/Fig-6]. Of the 32 samples with dual fungal growth, 14 showed coexisting growth of A. niger complex and C. albicans, eight showed A. flavus complex and A. fumigatus complex, six A. niger complex and A. fumigatus complex and four A. flavus complex and C. albicans. Fungi isolated from one sample with triple fungal growth were A. flavus complex, A. fumigatus complex and Penicillium and from other were A. flavus complex, A. fumigatus complex and C. tropicalis [Table/Fig-6,5 and 8].
Association between predisposing factors and positive cases of otomycosis.
Spectrum of fungal and bacterial isolates among patients.
| Fungal isolates | Number (%) of samples positive for fungi, n = 310 | Associated bacterial isolates (n) |
|---|
| Aspergillus niger complex | 186 (60) | Staphylococcus aureus | 41 |
| Klebsiella spp. | 10 |
| Pseudomonas aeruginosa | 9 |
| E. coli | 2 |
| Proteus spp. | 4 |
| Aspergillus flavus complex | 46 (14.8) | Staphylococcus aureus | 24 |
| Pseudomonas aeruginosa | 8 |
| Klebsiella spp. | 6 |
| Aspergillusfumigatus complex | 20 (6.5) | Pseudomonas aeruginosa | 10 |
| Candida albicansCandida kruseiCandida tropicalis | 10 (3.2)4 (1.3)2 (0.64) | Klebsiella spp. | 4 |
| Staphylococcus aureus | 2 |
| Penicillium | 4 (1.3) | Pseudomonas aeruginosa | 2 |
| Mucor | 2 (0.64) | Staphylococcus aureus | 1 |
| Trichophyton mentagrophyte | 2 (0.64) | -- | |
| Mixed fungal infections | With 2 fungi Aspergillus spp. : 46 (A. niger complex -20, A. fumigatus complex -14, A. flavus complex -12) Candida spp.: 18 | 32 (10.3) | Staphylococcus aureusKlebsiellaspp. Pseudomonas aeruginosa | 1753 |
| With 3 fungi Aspergillus spp.: 4 (A. fumigatus complex -2, A. flavus complex -2) Penicillium : 1 Candida tropicalis : 1 | 2 (0.64) | | |
| Total fungal isolates | 346 | Total bacterial isolates | 148 |
Proportion of samples with mixed fungal growth.
Distribution of fungal agents in samples with mixed fungal growth.
Microscopy and culture of fungal isolate.
Bacteria isolated from samples with mixed (dual) fungal growth are S. aureus, Klebsiella spp. and Pseudomonas aeruginosa. No bacteria was isolated from samples with triple fungal growth [Table/Fig-5].
Discussion
Otomycosis is a superficial mycotic infection of EAC, highly prevalent in tropical and subtropical regions of the world [2-4,13]. Many researchers reported this problem from India and other parts of world in the last few decades [2,4-10,14]. One of the important environmental factors which are responsible for increasing number of fungal infections of the ear is high content of suspended dust particles in the air. Fungi found abundantly on decaying plant matter, can be blown in the wind with soil particles and carried away by water vapours in the rainy season. This correlates well with higher rate of fungal infections during rainy season. Gurgaon, where the present study has been undertaken, is a city in the National Capital Region of India. Many projects are under construction from past few years in and around Gurgaon city which has increased the content of dust particles in the air to many folds. Ambient temperature varies from 8°C to 48°C. Relative humidity is high during rainy season, approx 75-80%. These conditions are very conducive for fungal growth [2]. Our study population mainly comprised of younger age groups which is in accordance with other studies from India and other countries [1, 4-6, 15]. No age group is immune to otomycosis (known fact) but the incidence appeared to be more in >15-35 yrs age group (66.3%) which goes well with other studies [2,5]. In comparison to females, more males were infected, this is in agreement with other studies [1, 4-6, 15]. Some studies have reported increased incidence in people of lower socioeconomic group [5,16] as observed in our study. Most of our study subjects were outdoor laborers and farmers who were exposed to fungal spores along with dust owing to their working conditions. Majority of the patients (96.6%) presented with unilateral involvement of the ear that has been reported by others in immunocompetent patients [8,9,15]. Bilateral involvement is more common in immunocompromised patients [6]. Right ear was involved predominantly [7,10,15]. Higher frequency of the right sided infection could be related to the dominant hand of study subjects.
Fungi can either be primary pathogens or superimposed on bacterial infections of the ear [14]. In our study we tried to exclude the patients with history of chronic otitis media, tympanic membrane perforations, ear surgery or other aural procedures in order to study the primary pathogenic status of fungal agents in causation of otomycosis. Present study was conducted on immunocompetent patients, as immunocompromised state and diabetes are known independent risk factors for otomycosis. Anatomical disposition of EAC provides ideal conditions for fungal and bacterial growth, as its small meatal inlet helps in retaining moist conditions in EAC [15]. Antimycotic and bacteriostatic properties of secretions of the apocrine and sebaceous glands (cerumen) protect healthy ear from invading organisms and fungal infections occur after the damage of these glands by bacteria or some other agents [17] so absence of cerumen increases the chances of fungal and bacterial infections as reported in a study [18] but researchers observed the growth of A. fumigatus and A. terreus on cerumen [19]. These observations support our findings as cerumen was present in most of study subjects. Presence of excessive cerumen in patients with poor personal hygiene favors germination of spores and conidia [5]. Scratching of ear canal in order to remove the cerumen and to get relief from itching can cause minor trauma in the skin of EAC which may get deposited by fungal spores that later on germinate and can cause fungal infection in presence of other predisposing factors. A popular myth that instillation of mustard oil can relieve itching and cure minor ailments of ear led the people of this area to adopt this habit. Our study revealed high association between otomycosis and instillation of mustard oil. This association was also reported in a study [4]. Strong association was also observed between otomycosis and use of ear drops. Normal bacterial flora of EAC is one of the host defense mechanisms against fungal infections. This mechanism is altered in patients using antibiotic ear drops thus making them prone to otomycosis [20].
Maximum patients presented with feeling of blocked ear (76.6%) followed by itching, pain and discharge. In a study otalgia was reported as the major symptom followed by otorrhoea and hearing loss [7]. Otoscopic findings varied from plug of black mycelial matted and brownish grey mycelial mass in Aspergillus otomycosis cases to soft epithelial debris and white curdy discharge in cases of dermatophytic and candidal otomycosis respectively.
Isolation rate of fungi from the suspected cases was 88.6% in the present study. Other studies reported 69%, 74.7% and 100% isolation rate [5,14,21]. Aspergillus spp. turned out to be predominant fungus isolated from 92.3% [Aspergillus spp. turned out to be predominant fungus isolated from 92.3% patients followed by Candida spp. (11.3%).] {186+46+20+34=286 patients/ samples with Aspergillus, 28600/310= 92.3%}. This finding is not different from other reports from India [4,5,16,22,23]. Though some studies reported Candida spp. as predominant organism [3, 24]. Aspergillus is a saprophytic mold and is one of the primary colonizers of manmade substrata. Rapid growth and production of a large number of small, dry and easily aerosolized conidia make it a significant contaminant with regard to air quality and potential human exposure related illnesses [16]. According to the rules of International Code of Botanical Nomenclature genus Aspergillus is classified into seven subgenera that are in turn sub-divided into several sections comprised of related species [25]. Mycological findings of the present study were based on morphological identification methods for detection of fungal agents especially Aspergillus spp., wherein the criteria of identification is based on recognition of asexual structures and their characteristics such as size, shape, arrangement etc. These characteristics are not helpful in clinical samples because of slow sporulation and aberrant conidiophore formation. Identification of Aspergillus spp. is of utmost importance as different species have different susceptibilities to antifungal drugs and it will affect the choice of appropriate antifungal therapy subsequently [26]. This problem can be solved to some extent by inoculating samples on suitable culture media and after a period of incubation, identifying the growth by examining LPCB mount of growth material [12] but we cannot rely completely on this conventional culture based phenotypic identification method for speciation and DNA sequence-based identification methods must be given a thought. Many researchers worked in this direction within the sections Fumigati, Terrei, Usti and Emericella. International group of experts recommended the comparative sequence analysis of Internal transcribed spacer region and sequence comparison of β tubulin region for identification of Aspergillus isolates to the section level and for species identification within the section respectively. Morphology based identification cannot identify species within a section so an alternative term ‘complex’ was proposed to ‘section’ to help support laboratories in reporting the members of genus, for instance ‘A. fumigatius complex’ [26]. Our study suggested A. niger complex as predominant fungi, similar to other studies [6,14]. Some studies reported A. fumigatus and A. flavus complex as most frequent fungi [5,13]. Due to non-specific presentation, sometimes it is difficult to identify infections with Candida spp. clinically [7] and laboratory identification is mandatory for proper treatment of patients. It was reported as predominant organism with otomycosis in immunocompromised hosts, in postoperative cavities and in infected middle ear earlier [6, 27]. We are reporting 34(11%) cases with infections due to mixed fungal flora. We tried but could not find such a large number of otomycotic cases with mixed infections in the literature except for a study from South India [28]. Some studies from India reported 1-6% cases of mixed infections [5, 14, 15, 29]. Mixed infections are generally scarce as fungal flora tends to inhibit the bacterial kind. These are difficult to cure and tend to re-occur probably due to formation of biofilms [21, 28, 30]. Candida has been known to form biofilms and studies are now suggesting biofilm formation by Aspergillus spp. [31-33]. In the past, mixed bacterial-fungal infections have been described for pneumonias and endocarditis [34, 35]. Mixed fungal infections that include fungal species from different genera have been reported in a study on patients being treated for onychomycosis of the toenail [36]. A case of otomycosis that included a mixture of A. niger and C. albicans was also reported [37]. Identifying mixed bacterial-fungal infections necessitates both antibacterial and antifungal therapy for complete cure and to prevent recurrence. In our study, no A. fumigatus was associated with S. aureus, which is in accordance with other study. This has been attributed to antibiotic activity of A. fumigatus against S. aureus [1]. In the past, many researchers have tried to elucidate the relationship of bacteria and fungi in mixed infections [38, 39]. English and Stanley [40] observed the interactions between A. niger, A. fumigatus, A. flavus, A. tereus and aural bacteria viz S. aureus and P. aeruginosa in their experiments. In their study researchers found the inhibitory effect of P. aeruginosa (by virtue of pigment production) and S. aureus (by production of antifungal toxic material) on the growth of A. fumigatus and A. tereus and promotional effect of A. fumigatus and A. tereus on the growth of bacteria [41]. In our study S. aureus, P. aeruginosa and Klebsiella spp. were dominant organisms which is in accordance with the findings of a study [14]. Otomycosis is a common problem in warm and humid area and among rural communities which uses home remedies for ear problems which in turn predispose them to otomycosis. Thus, educating them to not to follow such beliefs can suppress the problem among such communities.
Limitation
Though we tried to isolate and characterize fungal agents upto species level by conventional methods, there could be a scope to employ molecular methods for speciation of fungal isolates.
Conclusion
Rural communities with higher practice of self cleaning and using home remedies to get relief from ear ailments are at higher risk of otomycosis. Species like Aspergillus and Candida are the common culprits involved in such cases. There is a need to educate rural population about serious consequences of self cleaning and using home remedies for ear problems.