Antimicrobial Effect of Leaves of Phyllanthus niruri and Solanum nigrum on Caries Causing Bacteria: An In vitro Study
J Sunitha1, Swathy Krishna2, R Ananthalakshmi3, J Sathiya Jeeva4, AS Smiline Girija5, Nadeem Jeddy6
1 Reader, Department of Pathology and Microbiology, Thai Moogambigai Dental College, Chennai, Tamilnadu, India.
2 Student, Department of Oral Pathology, Thai Moogambigai Dental College, Chennai, Tamilnadu, India.
3 Reader, Department of Oral Pathology, Thai Moogambigai Dental College, Chennai, Tamilnadu, India.
4 Reader, Department of Oral Pathology, Thai Moogambigai Dental College, Chennai, Tamilnadu, India.
5 Reader, Department of Microbiology, Meenakshi Ammal Dental college, Chennai, Tamilnadu, India.
6 Professor and HOD, Department of Pathology and Microbiology, Thai Moogambigai Dental College, Chennai, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. J Sunitha, 27 Block, Flat No 2107, LIC Colony, Annanagar West Extension, Chennai-600101, Tamilnadu, India.
E-mail: sunijana@rediffmail.com
Introduction
Solanum nigrum and Phyllanthus niruri are common herbs which are indigeneous to India. Solanum nigrum commonly called ‘manathakkali Keerai’ in Tamil, forms an indispensable part of South Indian diet. Phyllanthus niruri (keezhanelli in Tamil) is a widely used medicinal plant, the leaves of which have been used extensively in Ayurveda and native medicine to cure various liver ailments. The herbs Solanumnigrum and Phyllanthus niruri have been found to be effective against numerous enteropathogens in various in vitro studies.
Aim
To assess and compare the antibacterial efficacy of the crude alcoholic extract of the leaves of Solanum nigrum and Phyllanthus niruri against five cariogenic organisms.
Materials and Methods
Standard strains of the micro-organisms were obtained from ATCC (American Type Culture Collection) and MTCC (Microbial Type Culture Collection) which comprised of Streptococcus mutans MTCC no. 890, Streptococcus oralis MTCC no 2696, Lactobacillus acidophillus MTCC no. 10307, Streptococcus sanguis ATCC no. 10556 and Streptococcus salivarius ATCC no. 13419. The organisms obtained were revived and lawn cultured on Trypticase Soy Agar-Blood Agar (TSA-BA) and de Man, Rogosa and Sharpe (MRS) agar media. The antibacterial effect of the dried and powdered leaves of Solanum nigrum and Phyllanthus niruri was tested using agar well diffusion method. The zones of inhibition obtained after incubation were measured and tabulated. The antibacterial activity for the two herbs was compared using the Mann-Whitney test.
Results
The antibacterial zones of inhibition obtained for the herb Solanum nigrum was in the range of 12.3-14.6 mm and ranged from 9.7-11.6 mm for the herb Phyllanthus niruri. When the zones of inhibition were compared for the herbs, Solanum nigrum showed significantly greater zones of inhibition compared to Phyllanthus niruri for the organisms Streptococcussanguis, Streptococcus salivarius, Streptococcus oralis and Streptococcus mutans (p-value<0.05).
Conclusion
The alcoholic extract of leaves of Solanum nigrum and Phyllanthus niruri showed significant antibacterial activity against cariogenic organisms, with Solanum nigrum being more anti-cariogenic than Phyllanthus niruri.
Cariogenic, Herbal, Micro-organisms, Zone of inhibition
Introduction
The oral cavity is colonised by a group of intricate micro-organisms which grow as a distinct oral biofilm, the dental plaque. This tooth adhering film causes the most common oral microbial infection known to man, called dental caries [1]. The prevalence of dental caries in India ranges from 60-65%, indicating its extensive occurrence [2].
Caries has a multifactorial aetiology with micro-organisms, tooth structure, refined sugars and time, playing vital roles in the disease process. Micro-organisms like Streptococcusmutans cause initiation of carious lesion on the tooth. The chief cariogenic micro-organisms are mainly Streptococcus mutans, Streptococcus sanguis, Streptococcus salivarius, Streptococcus mitis, Streptococcus sobrinus and Lactobacillus acidophilus [1]. Thus, inhibition of the growth of these micro-organisms can curb the incidence of dental caries. Streptococcus mutans antigens are also being studied extensively for use as a vaccine against dental caries [3].
Herbal medicines are becoming popular in the current era, with people preferring them over conventional drugs to treat ailments with the notion that they cause lesser adverse effects. Also, certain pathogens have developed resistance to the conventional antiseptics and antibiotic, stipulating their cautious use [4]. According to WHO strategies and guidelines proposed in 2002 for herbal medicines, any plant is considered to have therapeutic potential if a portion of the plant has been proved to have beneficial effects for the treatment of a disease through meticulous research [5,6]. Thus on going effort to find an anticariogenic agent which eliminates or inhibits the cariogenic organism from herbal sources would be a boon.
In India the application of herbs for treatment has been an integral part of our culture and continues to play a key role. It forms the basis of the origin of ancient medical sciences like Ayurveda and Siddha. Thus the use of indigenous herbs could provide an alternative to conventional treatment modalities [7]. Solanum nigrum or black berry night shade in English have been used as herbal remedy to cure various liver ailments, stomach disorders, asthma, whooping cough etc., [8].
Similarly Phyllanthus niruri commonly termed ‘stone breaker’ in English is a common weed, found in both cultivated fields and wastelands. It is considered to be antihepatotoxic, antihepatitis-B and an antidiabetic [9-11]. As per our knowledge there was no other studies done to evaluate the anticariogenic effect of Solanum nigrum and Phyllanthus niruri. We started this work with the hypothesis that the herbs Solanum nigrum and Phyllanthus niruri can inhibit the growth of cariogenic organisms. Thus the aim of the present study was to assess whether these herbs have antibacterial effect on cariogenic organisms Streptococcus mutans, Streptococcussanguis, Streptococcus oralis, Streptococcus salivarius and Lactobacillus acidophilus. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the herbal extracts were also determined.
Materials and Methods
The study was done in an experimental laboratory setting with an in vitro study design, for a period of three months in 2015 at Central Research Laboratory, MAHER University. The study was approved by the Institutional Ethical Committee, Meenakshi Ammal Denal College. Standard strains of the organisms were obtained from MTCC and ATCC. The organisms ordered were Streptococcusmutans MTCC no. 890, Streptococcus oralis MTCC no 2696 and Lactobacillus acidophilus MTCC no. 10307, Streptococcussanguis ATCC no. 10556, Streptococcus salivarius ATCC no. 13419. The organisms were revived and the sugar limited cultures were maintained at a pH of 7 throughout the stationary phase. The leaves of the two plants Solanum nigrum and Phyllanthus niruri were dried in shade and powdered using a blender. The resulting powder was then stored in air tight wide mouth containers. A 25 mg of herbal powders were dissolved in 75 ml ethyl alcohol (1:3 w/v) and extracted at room temperature. The solution was then placed in an orbital shaker and left for three days. The resulting solution was filtered using a whatman filter paper. The filterate was placed in a rotary evaporater. The powder obtained was weighed and 100 mg of the powder was mixed with 1 ml of DMSO (Dimethyl Sulphoxide) and stored in amber coloured bottles at a temperature of –20°C for further use [4].
The antibacterial susceptibility was tested by agar well diffusion method. Inoculums were prepared and adjusted to 0.5 McFarland turbidity standard guidelines [12]. The inoculum suspension from the nutrient broth was streaked uniformly on TSA-BA and MRS agar. Wells were cut into the lawn cultures with a sterile borer of well of 0.5 cm diameter in the pathogen inoculated media. A 50 µl of each extract were aseptically filled into the well. The plates were placed at room temperature to allow diffusion of extract into the agar for a period of one hour. It was then incubated for 24 hours at 37°C. The results were recorded by measuring the diameter of zone of inhibition at the end of 24-48 hours. A 0.2% chlorhexidine was used as a positive control, while DMSO medium without the herbal extracts was taken as a negative control. After incubation the clear zone around the wells was measured in mm and was recorded as the zone of inhibition. The test was done in triplicates and average values were recorded.
The MIC Determination and MBC determination was done using the broth dilution method [12]. A pure culture of the test organism was grown in the respective broth. A 100 µl of the nutrient broth was mixed with 100 µl of the herbal extract (1:1) in column 1 of the microtitration tray. After the herbal extract has been diluted, 100 µl volume of the diluted herbal extract from column 1 was added to each dilution vessel in column 2 using a multichannel pipette, 100 µl was then removed from column 2 and added to column 3. Thus serial dilution beginning from 100 µl to 50 µl, 25 µl, 12.5µl, 6.25 µl till 3.125 µl was performed. The inoculated, serially diluted herbal agent was then incubated for about 24 hours. The series of dilution vessels were then observed for microbial growth, indicated by turbidity in the bottom of the vessel. The last dilution tube that did not show growth corresponded to the MIC of the antimicrobial agent. The MIC dilution and two other dilutions of the herb were then plated to determine the Colony Forming Units (CFUs). The MBC (Minimum Bactericidal Concentration) value was determined as the lowest concentration at which 99.9% of the bacteria showed lysis [12].
The results were tabulated and since the data obtained was non parametric, Mann-Whitney test was used to compare the zones of inhibition of the two herbs.
Results
The results have been summarised in [Table/Fig-1,2,3,4,5,6,7 and 8]. The herb Phyllanthus niruri Labelled as ‘A’ (9.6 mm-11.6 mm) showed lesser antibacterial inhibition than Solanum nigrum (12.3 mm-14.6 mm) labelled as ‘B’. The results were statistically significant for Streptococcus sanguis, Streptococcus salivarius, Streptococcus oralis and Streptococcus mutans (p-value<0.05). Although for Lactobacillus acidophilus, the inhibitory effect of Solanum nigrum was more than Phyllanthus niruri, the p-value>0.05 indicating that it is not statistically significant [Table/Fig-3]. The MIC and MBC were also found to be least. The MIC for Lactobacillus acidophilus (25 and 50 mg/ml) for both the herbs Solanum nigrum and Phyllanthus niruri respectively, indicating that these herbs were effective antimicrobials even in lower concentrations [Table/Fig-9,10].
Effect of manathakkali (Solanum nigrum) leaves extract on cariogenic micro-organisms, zone of inhibition obtained are shown in the table. The test was done in triplets and the mean was calculated.
| Organisms | 1st test | 2nd test | 3rd test | Mean |
|---|
| Streptococcus sanguis | 14 | 15 | 14 | 14.3 |
| Streptococcus salivarius | 15 | 14 | 14 | 14.3 |
| Streptococcus oralis | 14 | 14 | 15 | 14.3 |
| Streptococcus mutans | 15 | 15 | 14 | 14.6 |
| Lactobacillus acidophilus | 12 | 13 | 12 | 12.3 |
Effect of alcoholic extract of Keezhanelli leaves (Phyllanthus niruri) against cariogenic micro-organisms, zone of inhibition obtained are shown in the table.
| Organisms | 1st test | 2nd test | 3rd test | Mean |
|---|
| Streptococcus sanguis | 12 | 11 | 12 | 11.6 |
| Streptococcus salivarius | 10 | 10 | 9 | 9.6 |
| Streptococcus oralis | 12 | 12 | 11 | 11.6 |
| Streptococcus mutans | 12 | 12 | 10 | 11.3 |
| Lactobacillus acidophilus | 12 | 11 | 10 | 11 |
Mann-Whitney test to compare between the zones of inhibition of the herbs Solanum nigrum and Phyllanthus niruri.
| Herb | N | Mean Rank | p-value |
|---|
| Streptococcus sanguis | Manathakkali | 3 | 5.00 | 0.043 |
| Keezhanelli | 3 | 2.00 |
| Streptococcus salivarius | Manathakkali | 3 | 5.00 | 0.043 |
| Keezhanelli | 3 | 2.00 |
| Streptococcus oralis | Manathakkali | 3 | 5.00 | 0.043 |
| Keezhanelli | 3 | 2.00 |
| Streptococcus mutans | Manathakkali | 3 | 5.00 | 0.043 |
| Keezhanelli | 3 | 2.00 |
| Lactobacillus acidophillus | Manathakkali | 3 | 4.67 | 0.105 |
| Keezhanelli | 3 | 2.33 |
(p<0.05 then significant).
Manathakkali or Solanum nigrum
Keezhanelli or Phyllanthus niruri
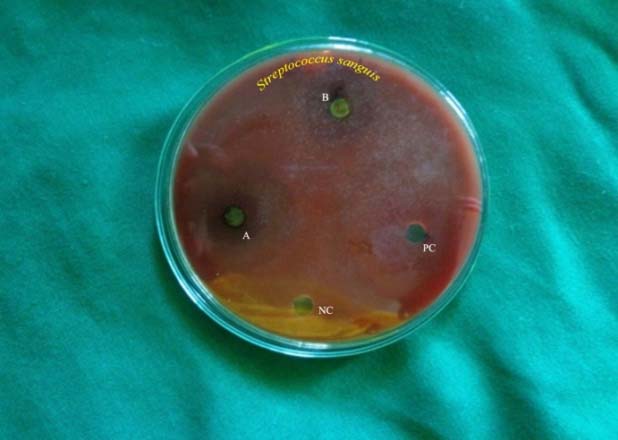
Antibacterial zones of inhibition against Streptococcus sanguis on TSA-BA. NC-Negative control, B-Solanum nigrum (14.3 mm), PC-Positive control, A-Phyllanthus niruri (11.6 mm)
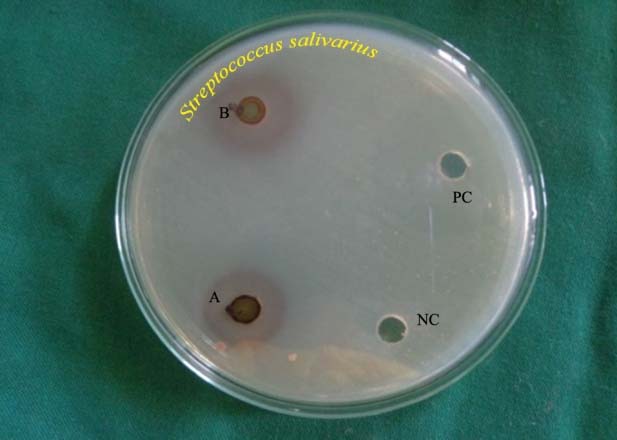
Antibacterial zones of inhibition against Streptococcus salivarius on TSA. NC-Negative control, B-Solanum nigrum (14.3 mm), PC-Positive control, A-Phyllanthus niruri (9.6 mm)
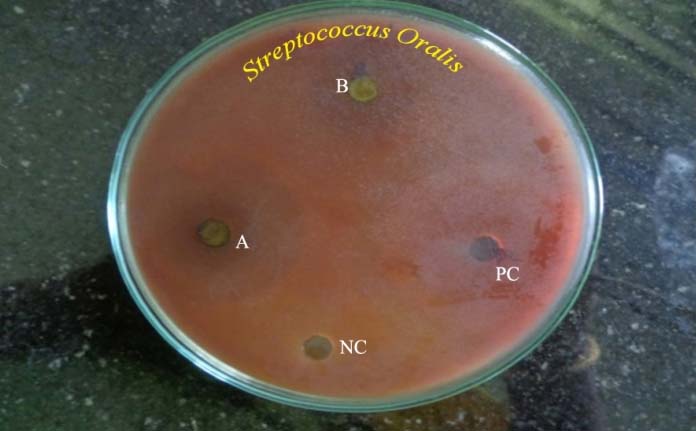
Antibacterial zones of inhibition against Streptococcus oralis on TSA-BA. NC- Negative control, B-Solanum nigrum (14.3 mm), PC- Positive control. A-Phyllanthus niruri (11.6 mm)
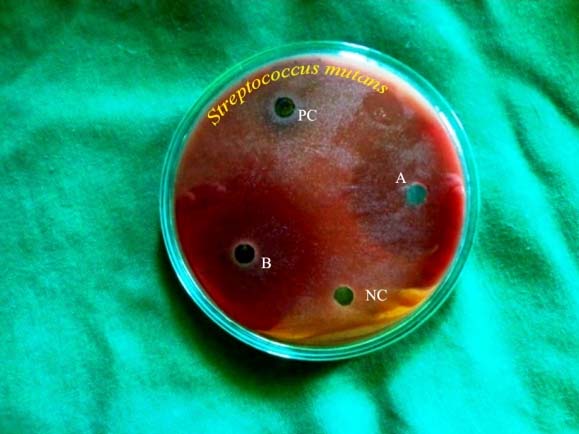
Antibacterial zones of inhibition against Streptococcus mutans on TSA- BA. NC- Negative control, B-Solanum nigrum (14.6 mm), PC- Positive control, A- Phyllanthus niruri (11.3 mm)
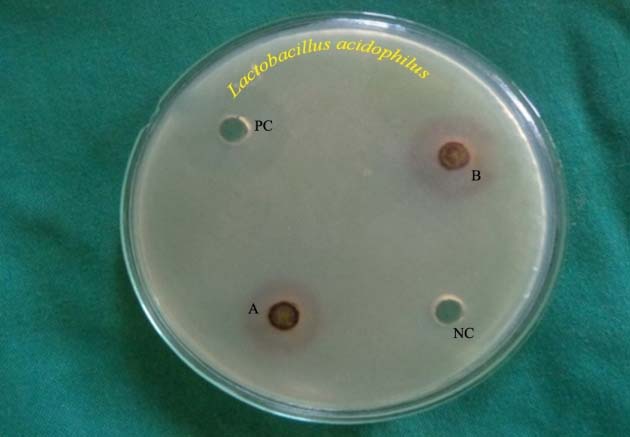
Antibacterial zones of inhibition against Lactobacillus acidophilus on MRS agar. NC-Negative control, B-Solanum nigrum (12.3 mm), PC- Positive control, A-Phyllanthus niruri (11 mm)
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration for Solanum nigrum.
| MIC values (mg/ml) | MBC values (mg/ml) |
|---|
| Lactobacillus | 25 | 50 |
| Streptococcus mutans | 100 | 200 |
| Streptococcus oralis | 50 | 100 |
| Streptococcus salivarius | 100 | 200 |
| Streptococcus sanguis | 100 | 200 |
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration for Phyllanthus niruri.
| MIC values (mg/ml) | MBC values (mg/ml) |
|---|
| Lactobacillus | 50 | 100 |
| Streptococcus mutans | 100 | 200 |
| Streptococcus oralis | 100 | 200 |
| Streptococcus salivarius | 100 | 200 |
| Streptococcus sanguis | 100 | 200 |
Discussion
In the Indian subcontinent herbal medicines are widely used and very often are the only treatment option for more than 70 percent of the population, particularly in rural areas. Over the years the potential chemotherapeutic property of herbal drug and its ability to counteract the antibiotic resistance has led to numerous innovation using herbal molecules [13]. Phyllanthus niruri and Solanum nigrum are indigenous herbs which have been used in India for treatment of various ailments [9].
The present in vitro study showed that the herbs Solanum nigrum and Phyllanthus niruri have an inhibitory effect on the growth of cariogenic organisms Streptococcus mutans, Streptococcussanguis, Streptococcus salivarius, Streptococcus oralis and Lactobacillus acidophilus which were cultured in their respective mediums. Any agent showing a zone of inhibition of more than 3 mm is considered to be an effective antimicrobial [12]. The result of our study showed both the herbs Solanum nigrum and Phyllanthus niruri had antimicrobial inhibitory zones ranging from 9.6-14.6 mm [Table/Fig-1,2], indicating that both the herbs canrestrict the growth of cariogenic organisms. Further when the zones of inhibition of the two herbs Solanum nigrum and Phyllanthus niruri were compared using Mann-Whitney test, the antibacterial activity of Solanum nigrum was found to be more than Phyllanthus niruri. In the Mann-Whitney test the results were statistically significant for Streptococcus sanguis (p<0.043), Streptococcus salivarius (p<0.043), Streptococcusoralis (p<0.043) and Streptococcus mutans (p<0.043) [Table/Fig-3]. Thus we can infer from the study that the herb Phyllanthus niruri and Solanum nigrum have inherent antimicrobial property. The MIC values of the herbs was lowest for Lactobacillus acidophilus (A=50 mg/mm, B=25 mg/ml) and Streptococcus oralis (B=50 mg/ ml) [Table/Fig-9,10] than for the other three organisms taken in the study, indicating that the herbs are potent antimicrobials on these two oral pathogens even at very low concentration.
Njoroge AD et al., showed that the in vitro antimicrobial efficacy of Phyllanthus niruri extracted in water, methanol and dichloromethane were bacteriolytic on various pathogenic bacteria (Bacillus pumilus, Staphylococcus aureus, Bacillus subtilis, Micrococcus luteus, Escherichia coli and Klebsiella pneumonia) and on fungus Candida albicans. The antibacterial action observed against the pathogens Bacillus pumilus with methanolic and dichloromethane extracts exhibited wider zones of inhibition measuring 18-20mm [14]. Obiagwu IN et al., did a similar study using different concentrations of ethanolic and aqueous extracts of Phyllanthus niruri on Escherchiacoli, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa and Klebsiella aerogenes. Pseudomonas aeruginosa was found to be most susceptible among the ethanolic extracts [11]. The MIC obtained for Escherchia coli was 50 mg/ml which is similar to the MIC value obtained for Lactobacillus acidophilus in our study. The antimicrobial activity was more for the methanolic extract than for aqueous extract. Since organic solvents are better than aqueous extract proved through previous studies, probably due to the fact that active components readily dissolve in solvent compared to water, so in our study we used ethanolic extract of the leaves of Solanum nigrum and Phyllanthus niruri.
The antibacterial effect of dried powdered Solanum nigrum fruits which were extracted in different organic solvents (Petroleum ether, chloroform, Dichloromethane, ethyl acetate, acetone, methanol) and water were evaluated against the pathogens Micrococcusluteus, Staphylococcus aureus, Salmonella typhi, Eschericha coli and fungus Candida albicans. Herbs which were extracted by using more polar solvents like methanol (7-14 mm zone of inhibition) had significant antimicrobial activity compared to those extracted with less polar solvents (3.6-9.8 mm) [15]. A similar study was done by Kasa P et al., who investigated the ethanolic and methanolic extracts of Solanum nigrum against pathogenic bacteria like Bacillussubtilis, Escherichia coli, Klebsiella pneumonia and Pseudomonas aeruginosa. The whole plant, stem and berries were individually extracted with the organic solvent and evaluated. Whole plant extract showed potential antibacterial activity than stem and berries [4]. Sridhar TM et al., evaluated the solvent extracts of seeds, leaf and roots of Solanum nigrum using various organic solvents like ethanol, methanol, ethyl acetate, diethyl ether, chloroform and hexane. Among the extracts, ethyl acetate extracts obtained from the seeds of Solanum nigrum were shown to exhibit significant activity against enteric pathogens Proteus vulgaris, Klebsiella and Bacillus subtilis [16].
Yogananth N et al., studied the antibacterial effect of Solanumnigrum against eight pathogenic organisms namely Enterococcus faecalis, Escherchia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Shigella flexneri, Salmonella typhi, Staphylococcus aureus and Vibrio cholera. The ethanolic extract was having significant antibacterial activity against all pathogens excluding Staphylococcus aureus and Pseudomonas aeruginosa [17]. Our study was the first such attempt to study the effect of these herbal leaf extracts of Solanum nigrum and Phllanthus niruri on caries causing pathogens.
Thus the present study confirms that the herbs Solanum nigrum and Phyllanthus niruri are potential anticaries agents. Numerous plants have been used to extract biomolecules and have been incorporated in toothpastes for use in oral care therapy [18-20]. The anticariogenic potential of the herbs in our current study, once proven through research can be incorporated into products for oral care.
Limitation
The limitation of this research include- it was not performed with various types of extract at different concentration, and studied only the alcoholic extracts of the herbs. This was an in vitro study, since the in vivo environment is unique, we cannot anticipate the same results when the herb is used in vivo settings. The present study used only the leaves of the herbs; the other parts of the plant like the seeds, stem, and fruits should also be explored for their antibacterial potential. The functional activity of the herbs Solanumnigrum and Phyllanthus niruri should be interpreted by cell culture studies as well and the molecular mechanisms of action should be elucidated.
Conclusion
Hence, we can conclude from the present study that Solanumnigrum and Phyllanthus niruri are potent antibacterial agents against Streptococcus mutans, Streptococcus sanguis, Streptococcus salivarius, Streptococcus mitis, and Lactobacillus acidophilus. When the activity of the two herbs were compared Solanumnigrum showed greater activity than Phyllanthus niruri. This in vitro study showed that the herbs have substantial anticariogenic potential, further research can lead to isolation of the key molecules responsible for the antibacterial action.
Conflict of interest: No conflict of interest has been reported for the same.
(p<0.05 then significant).
Manathakkali or Solanum nigrum
Keezhanelli or Phyllanthus niruri
[1]. Sivapathasundharam B, Raghu AR, Sivapathasundharam B, Rajendran R Editors, Microbiology of dental cariesShafer’s textbook of Oral Pathology6th edElsevier publications:438-43. [Google Scholar]
[2]. Grewal H, Verma M, Kumar A, Prevalence of dental caries and treatment needs amongst the school children of three educational zones of urban Chennai, IndiaIndian J Dent Res 2011 22(4):517-19. [Google Scholar]
[3]. Shivakumar KM, Vidya SK, Chandu GN, Dental caries vaccineIndian J Dent Res 2009 20:99-106. [Google Scholar]
[4]. Kasa P, Aluru S, Kishori B, In vitro antibacterial activity in the extracts of SolanumnigrumIndian Streams Research Journal 2012 2(7):1-4. [Google Scholar]
[5]. World Health Organization 2002WHO Traditional medicine strategy 2002-2005, World Health Organization [Google Scholar]
[6]. Sofowora A, Ogunbodede E, Onayade A, The role and place of medicinal plants in the strategies for disease preventionAfrican Journal of Traditional, Complementary, and Alternative Medicines 2013 10(5):210-29. [Google Scholar]
[7]. Saranya SR, Krishna PJ, Singh RK, Gaanappriya M, Dhivya E, Rajasekar S, Screening and phytochemical analysis of pharmacologically active compounds from abutilon indicum and phyllanthus niruri and assessing their in vitro anti microbial activity against pathogensIntl J of Adv Biotec and Res 2013 4(4):496-504. [Google Scholar]
[8]. Gupta S, Sarethy IP, Gabrani R, Solanum nigrum:current perspectives on therapeutic propertiesAltern Med Rev 2011 16(1):496-504. [Google Scholar]
[9]. Paithankar VV, Raut KS, Charde RM, Vyas JV, Review Article Phyllanthus niruri:A magic HerbResearch in Pharmacy 2011 1(4):1-9. [Google Scholar]
[10]. Nivetha SA, Roy DB, Antimicrobial studies on ethanolic extracts of PhyllanthusniruriJ Chem Pharm Res 2015 7(6):103-06. [Google Scholar]
[11]. Obiagwu IN, Okechalu OB, Njoku MO, Studies on antibacterial effect of the leaves of phyllanthus niruri on some enteric pathogensNig J Biotech 2011 23:22-27. [Google Scholar]
[12]. Forbes BA, Sahm DF, Weissfeld AC, Laboratory methods and strategies for Antimicrobial Susceptibility Testing. 2007 ed Bailey & Scott’s Diagnostic Microbiology12th edSt. Louis, MOMosby Elsevier:187-214. [Google Scholar]
[13]. Doughari JH, Human IS, Bennak S, Ndakidemi PA, Phytochemicals as chemotherapeutic agents and antioxidants:Possible situation to control of antibiotic resistance verocytotoxin producing bacteriaJ. Med. Plants Res 2009 3(11):839-48. [Google Scholar]
[14]. Njoroge AD, Anyango B, Dossaji SF, Screening of phyllanthus species for antimicrobial propertiesChemical Sciences Journal 2012 6:56-60. [Google Scholar]
[15]. Abbas K, Niaz U, Hussain T, Saeed MA, Javaid Z, Idrees A, Antimicrobial activity of fruits of Solanum nigrum and solanumxanthocarpumActa Poloniae Pharmaceutica ñ Drug Research 2014 71(3):415-21. [Google Scholar]
[16]. Sridhar TM, Josthna P, Naidu CV, In vitro antibacterial activity and phytochemical analysis of Solanum nigrum (linn.)- an important antiulcer medicinal plantJournal of Experimental Sciences 2011 2(8):24-29. [Google Scholar]
[17]. Yogananth N, Buvaneswari S, Muthezhilan R, Larvicidal and antibacterial activities of different solvent extracts of Solanum nigrumGlobal Journal of Biotechnology & Biochemistry 2012 7(3):86-89. [Google Scholar]
[18]. Jamil A, Shahid M, Khan MUH, Asharf M, Isolation of antifungal proteins and peptidesPak J Bot 2007 39(1):211-17. [Google Scholar]
[19]. Iwu MW, Duncan AR, Okunji CO, Janick J, New antimicrobials of plant originPerspectives on New Crops and New Uses 1999 Alexandria, VAASHS Press:457-462. [Google Scholar]
[20]. Koo H, Jeon J, Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilmsAdvances in Dental Research 2009 21:63-68. [Google Scholar]