Ameloblastoma is one of the common benign neoplasms of odontogenic epithelial origin, which is characterised by aggressive biological behaviour, local invasion, destruction of jaw bones, and high recurrence rate [1]. Because of these features, AB has been a matter of debate for inclusion under the category of low grade malignant tumours [2]. Very few benign tumours possess this characteristic feature of local invasion. Many studies have been done in an attempt to clarify the invasion phenomenon in ABs [3-10]. However, the exact molecular mechanism of invasion in ABs has not been well elucidated. Thus, identification of the prognostic markers of the AB’s biologic behaviour is of considerable importance to determine the most appropriate therapeutic approach and establish the prognosis of the patients.
Focal Adhesion Kinase (FAK) is a cytoplasmic tyrosine kinase molecule closely associated with the membrane. FAK plays an important role in integrin-mediated signal transductions and also contributes to the growth factor receptor signaling [11]. Recent studies have shown that FAK is an essential mediator of cell adhesion, proliferation, survival, angiogenesis and migration [12]. Kinase activity and molecular interaction are the important factors that govern the FAK-mediated signaling. For example, after integrin clustering, FAK binds to the cytoplasmic tail of β-integrin and results in phosphorylation of FAK tyrosine Y397 [13]. Such autophosphorylation of Y397 generates a high affinity binding site for Src. The interaction with Src results in ERK MAPK stimulation, which initiates cell proliferation [14]. Once further phosphorylated by Src, FAK can also recruit Jun N-terminal Kinase (JNK) to focal adhesion sites and the JNK pathway is also implicated in promoting FAK-initiated signals controlling tumour cell invasion [15]. In our previous study, we have reported a strong expression of FAK in the keratocystic odontogenic tumour, which is considered as a locally invasive benign neoplasm. In contrast, non invasive and non aggressive cysts like dentigerous and radicular cysts had shown weak expression of FAK [16].
With aforementioned aspect of FAK in mind, we have hypothesised that being locally invasive in nature; AB would show increased expression of FAK in the epithelial component. To prove this hypothesis, we compared FAK expression between AB and inactive odontogenic epithelial islands seen in DF.
Materials and Methods
Tissue samples: The present study was an observational in vitro study. The tissue samples were collected from the files of the Department of Oral Pathology and Microbiology, Dr. DY Patil Dental College and Hospital, Pune, India. The cases of AB and DF received between the year 2000 and March 2015 were reviewed. Total 34 cases of AB and 17 cases of DF were retrieved for immunohistochemical analysis. Cases were reviewed and selected based on the established guidelines [17]. Paraffin embedded specimens with adequate tumour tissue and odontogenic epithelial islands formed the case inclusion criterion. Biopsy samples showing inadequate odontogenic epithelial components were excluded from the present study. Patient details including age, gender, anatomic site, radiological findings, treatment, and case summaries were retrieved from the requisition forms and case history records. Also, patients without proper clinicopathological records mentioned above were excluded from the study.
Immunohistochemistry: Immunohistochemical staining was performed on 5 μm thick formalin fixed paraffin embedded sections. Sections were deparaffinized in xylene and then rehydrated in graded ethanol solutions. Antigen retrieval was accomplished by microwaving at 99°C in 10 nM of citrate buffer (pH 6) for 20 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 minutes, and sections rinsed in 0.05 M Tris-buffered saline (five minutes, two times) before immersing in blocking solution (Dako Corporation, Carpinteria, CA, USA) for 20 minutes at room temperature. Sections were incubated with the following primary mouse antibody FAK {C-20; sc558, SANTA CRUZ BIOTECHNOLOGY, INC, USA; dilution 1:100} for 30 minutes at room temperature. Immunoreactions were performed using the Envision Kit (Dako). The antigenic sites were visualized using diaminobenzidine (DAB) substrate chromogen (Dako) and counterstained with Mayer’s haematoxylin. Known positive sections of oral squamous cell carcinoma were used as positive controls. In addition, positive labeling for FAK in endothelial cells of AB and DF specimens served as internal positive controls. For negative control, sections were treated as above but without the primary antibody. All the control sections were found to be negative.
Immunohistochemical assessment: A semiquantitative method was used to score the FAK expression in the odontogenic epithelium of AB and DF, as previously described by Soini Y et al., Scoring was based on: (a) the intensity of the immunostaining in the ameloblast like and stellate reticulum like cells (0=absent, 1=weak, 2=moderate, 3=strong, and 4=very strong); and (b) the percentage of positive malignant cells (0=0% positive cells, 1= < 25% positive cells, 2=25–50% positive cells, 3=50–75% positive cells, and 4= >75% positive cells) [18]. The final immunostaining score was determined by the sum of (a) and (b). Final scores ranged from 0 to 8 (0=absent, 1–4=weak, and 5–8=strong).
Statistical Analysis
Data collection was conducted using the Microsoft Office Excel package and processed with the SPSS 16.0 software package (SPSS Inc. Chicago, IL) for the statistical analysis. The expression of FAK in different parameters was analysed using chi-square test or Fisher’s-exact test when the assumptions of the chi-square test were not met. A p-value < 0.05 were considered statistically significant.
Results
Clinical characteristics: The ages of the patients with AB ranged from 8 to 67 years (mean 34.26 ± 15.21 years). Females (58.82%) were more commonly affected than males (41.17%) with male to female ratio of 1:1.4. The most common location of the tumour was posterior mandible (24 cases) followed by parasymphyseal region (six cases), anterior maxilla (three cases), and posterior maxilla (one case). There were 21 cases of follicular variants (18 conventional, one granular and two acanthomatous), five of plexiform and eight of unicystic AB (one luminal, four luminal + intraluminal, two luminal + mural and one luminal + intraluminal + mural) Most common radiological appearance was multilocular radiolucency (26) while eight lesions (unicystic AB) showed unilocular radiolucency. Five recurrent cases were reported in the present study.
Total 25 DF tissues were obtained during surgical removal of impacted mandibular third molars. There were 12 males and 13 females. Their ages ranged from 17 to 32 years (mean 22.24 years). On radiological examination, the follicular space ranged from 1.5 mm to 2.5 mm with a mean of 2.18±0.31. Odontogenic epithelial islands were counted per specimen, which ranged from 2 to 6 (mean 3.88±1.03) and 4 to 15 cells were present in each epithelial island.
FAK expression: The expression of FAK was classically present in the cytoplasm of odontogenic epithelium as well as in the endothelial cells, which act as an internal positive control. In AB, ameloblast like cells showed strong expression of FAK in all the cases (34) [Table/Fig-1]. Stellate reticulum like cells showed strong expression in 12 cases, weak in five and negative in 17 cases [Table/Fig-1]. The differences in the expression of FAK in ameloblast like and stellate reticulum like cells were statistically significant (p < 0.0001) [Table/Fig-2]. We also found nuclear expression of FAK in 11 (32.35%) cases of AB. Out of these 11 cases, five were recurrent cases while six were non recurrent solid/multicystic cases. The nuclear expression of FAK was evident in both ameloblast like and stellate reticulum like cells. All the odontogenic epithelial islands in DF showed negative expression for FAK [Table/Fig-3].
Photomicrographs showing positive focal adhesion kinase expression in ameloblast like and stellate reticulum like cells of follicular (a), plexiform (b) and unicystic (c) ameloblastoma. However, some cases showed positive focal adhesion kinase expression in ameloblast like cells and negative in stellate reticulum like cells of follicular (d), plexiform (e) and unicystic (f) ameloblastoma. (Magnification 40 X; Immunohistochemistry).
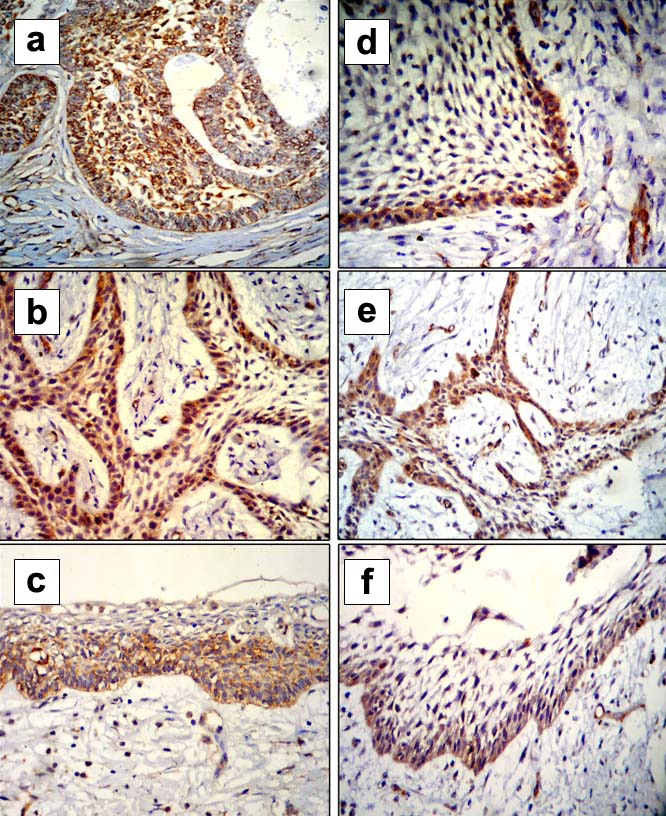
Focal adhesion kinase expression in ameloblast like and stellate reticulum like cells of ameloblastoma.
| Ameloblastoma | n | Fak expression | Chi-square value | p-value |
|---|
| Negative | Weak | Strong |
|---|
| Peripheral/Basal | 34 | 0 | 0 | 34 | 32.52 | <0.0001 |
| Stellate cells | 34 | 17 | 5 | 12 |
Statistical test: Fisher’s-exact test
Photomicrograph showing negative expression of focal adhesion kinase in inactive odontogenic epithelial islands of dental follicle. (Magnification 40X; Immunohistochemistry).
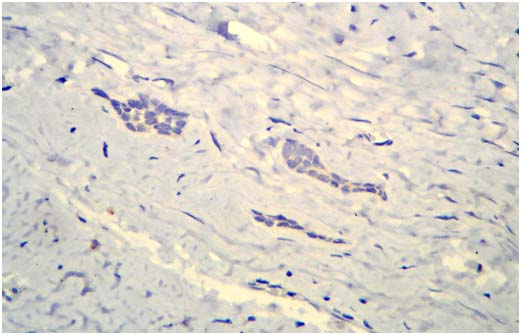
In all the cases of solid/multicystic AB, unicystic AB, recurrent AB and non recurrent AB, basal cells showed strong expression of FAK [Table/Fig-1]. Hence, statistical analysis for comparison of the expression among the above groups was not indicated. However, FAK expressions in stellate reticulum like cells of solid/multicystic AB vs. unicystic AB (p=0.7133) and recurrent AB vs. non recurrent AB (p=0.36) did not show any significant differences [Table/Fig-4].
Expression of focal adhesion kinase in different types of ameloblastoma.
| Parameter | n | FAK expression in ameloblast like cells | p-value | Fak expression in stellate reticulum like cells | p-value |
|---|
| Negative | Weak | Strong | | | |
|---|
| Ameloblastoma | 34 | 0 | 0 | 34 | * | 17 | 5 | 12 | 0.0017# |
| Dental Follicle | 17 | 17 | 0 | 0 | 17 | 0 | 0 |
| Solid/Multicystic | 26 | 0 | 0 | 26 | * | 12 | 4 | 10 | 0.7133 |
| Unicystic | 8 | 0 | 0 | 8 | 5 | 1 | 2 |
| Recurrent | 5 | 0 | 0 | 5 | * | 2 | 0 | 3 | 0.36 |
| Non recurrent | 29 | 0 | 0 | 29 | 15 | 5 | 9 |
All cases showed either strong or negative expression in each group; hence statistical analysis was not indicated. Statistical test: Fisher’s-exact test.
Statistically significant result.
Statistically significant results were observed in the expression of FAK in stellate reticulum like cells and DF (p=0.0017). Similarly, all AB cases showed strong expression of FAK in ameloblast like cells and negative expression in all the cases of DF [Table/Fig-4].
Discussion
AB is a benign odontogenic neoplasm that may exhibit aggressive biological behaviour as evidenced by its rapid growth and high recurrence rate following initial surgical resection. Currently, the only therapy for AB is surgical, and adjunctive treatment modalities are needed to mitigate tumour growth and to reduce the need for extensive and disfiguring surgeries. Many studies have identified markers expressed by AB and these lend insight to our understanding of tumour progression. It is important to determine which markers hold the greatest promise for a future therapeutic target of this benign neoplasm in order to improve the treatment options. Various aspects of AB like cell survival, proliferation, angiogenesis etc. have been widely studied in the literature using immunohistochemical markers. However, local invasion, which is the most important aspect of AB, has not been given much attention. The invasion related markers investigated in AB are Syndecan-1, α5β1 integrin, Podoplanin, metallothionein, Cyclin D1, MMP-1, -2, -9, PTHrP, β-catenin etc., [3-10].
FAK was originally identified as a Src oncogene substrate but recently has also been found to be activated by the SRC family kinases like PLC, SOCS, GRB7, PI3K, lysophosphatidic acid [19,20]. FAK responds to extracellular matrix interactions and through its mechanosensor capacity directs cell migration and invasion [19]. Similarly, FAK also translocates lipid raft components of the cell causing cell motility thereby enabling the microtubule-cortical receptor stabilization that is essential for directed cell movement. Moreover, FERM domain of the FAK, the main molecule responsible for chemosensor function of extracellular signals, provides receptor cross-talk and modulates signal transduction to the nucleus [21]. FAK’s FERM domain is also responsible for FAK nuclear translocation enabling FAK-mediated p53 degradation and resultant increased cell survival and proliferation [22]. Thus, FAK role in cell survival, proliferation, migration, invasion and angiogenesis makes it an attractive therapeutic target [19,23].
In this preliminary study, there was a statistically significant difference in the FAK expression in ameloblast like cells and stellate reticulum like cells. Higher expression in ameloblast like cells indicates its invasive and aggressive nature of the cells as compared to stellate reticulum like cells. In all the cases of solid/multicystic AB, unicystic AB, recurrent AB and non-recurrent AB, basal cells showed strong expression of FAK. Hence, statistical analysis for comparison among these above-mentioned groups was not indicated. However, FAK expression in stellate reticulum like cells of solid/multicystic AB vs. unicystic AB and recurrent AB vs. non recurrent AB did not show any significant differences (p=0.7133 and 0.36 respectively). Since no studies till date reported the expression of FAK in AB, comparison of aforementioned results with previous literature is not possible. However, in a recent study, we have reported a strong expression of FAK was in the majority of the cases of keratocystic odontogenic tumours, which is considered as locally aggressive neoplasm like AB. In contrast, dentigerous cyst showed negative expression in majority of the cases. Orthokeratinized odontogenic keratocyst is known for its less aggressive behaviour and low recurrence rate. In this regard, this cyst showed negative and weak expression in majority of the cases. Similarly, Radicular cyst, which is an inflammatory cyst with less aggressive behaviour, showed negative expression in 20 (80%) cases and weak expression in four (16%) of the cases [16].
In the present study, FAK expression was studied in the epithelial remnants of DF. These DFs were obtained during the extraction of impacted mandibular third molars. They were mainly composed of fibrocellular connective tissue stroma along with inactive odontogenic epithelial islands and reduced enamel epithelium. Both the sources of epithelium represent quiescent and mature cells, which have performed their functions and remain dormant in the connective tissue for indefinite period of time. Therefore, the epithelium of the DF was used as control to compare with the islands and cords of AB. In the present study, we found statistically significant differences in the expression of FAK in stellate reticulum like cells and DF (p=0.0017). Similarly, all the AB cases showed strong expression of FAK in ameloblast like cells and negative expression in all the cases of DF. This is the first study to report the expression of FAK in the epithelium of AB and DF; hence a comparison with the previous literature is not possible.
Translocation of the FAK to the nucleus is well known phenomenon, which take place under variety of stimuli. FAK presence in the nucleus promotes ubiquitination of p53 and degradation and thus causes enhance cell survival under stress conditions [24,25]. In the present study, we found nuclear expression of FAK in 11 cases of AB. Moreover, five of these were reported in the recurrent AB cases. We believe that nuclear FAK-mediated cell survival mechanism might be acting in AB; however, future studies are needed to authenticate the present finding.
Limitation
Lack of follow up data is the limitation of the present study, which could have provided information about the role of FAK as a marker of aggressiveness in AB. We recommend future studies on larger sample size to authenticate the role of FAK in tumourgenesis of AB. It would be interesting to know the FAK expression in different histopathological variants of ameloblastoma, which is not carried out in the present study due to limitation of the sample size. Future molecular and genetic studies are recommended to elucidate the downstream and upstream regulators of FAK in AB, which will further impact the future development of targeted therapy.
Conclusion
We have reported the expression of FAK in AB, which we believe is the first study of its kind. There was positive FAK expression in AB and negative in odontogenic epithelial islands of DF suggesting that FAK could be responsible for local invasiveness of AB. We did not find any significant differences in FAK expression in solid/multicystic vs. unicystic and recurrent vs. non recurrent AB. Thus, we conclude that FAK could act as a potential therapeutic target in AB. We recommend future studies on larger sample size to substantiate our findings and gain more insight into the role of FAK in predicting the biological behaviour of AB.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Statistical test: Fisher’s-exact test
*All cases showed either strong or negative expression in each group; hence statistical analysis was not indicated. Statistical test: Fisher’s-exact test.
#Statistically significant result.