Case Report
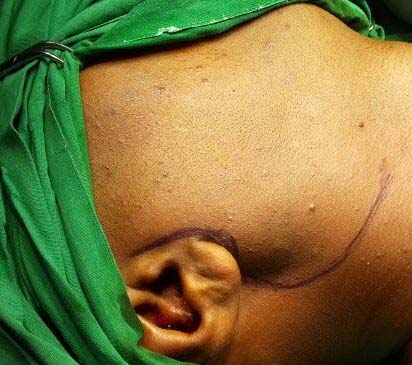
An 18-year-old Indian male presented with a progressive swelling below right ear since nine years. History of present illness revealed no associated complaints like pain or itching over the swelling, dry mouth, weight loss or night sweating. Localized examination revealed a 5 x 3 cm, firm, non tender, partially mobile, subcutaneous nodule extending from right mandibular angle upto right parotid region [Table/Fig-1]. Overlying skin was normal. General physical examination showed no facial asymmetry, no cervical, axillary or inguinal lymphadenopathy and no hepatosplenomegaly.
Right subcutaneous swelling extending from angle of right mandible to right parotid region.
Laboratory work up revealed a normal white blood cell count without eosinophilia (4%). Urine analysis and renal function test were within normal limits. Serum IgE level (59.30 IU/ml) and absolute peripheral eosinophil count (280/mm3) were normal.
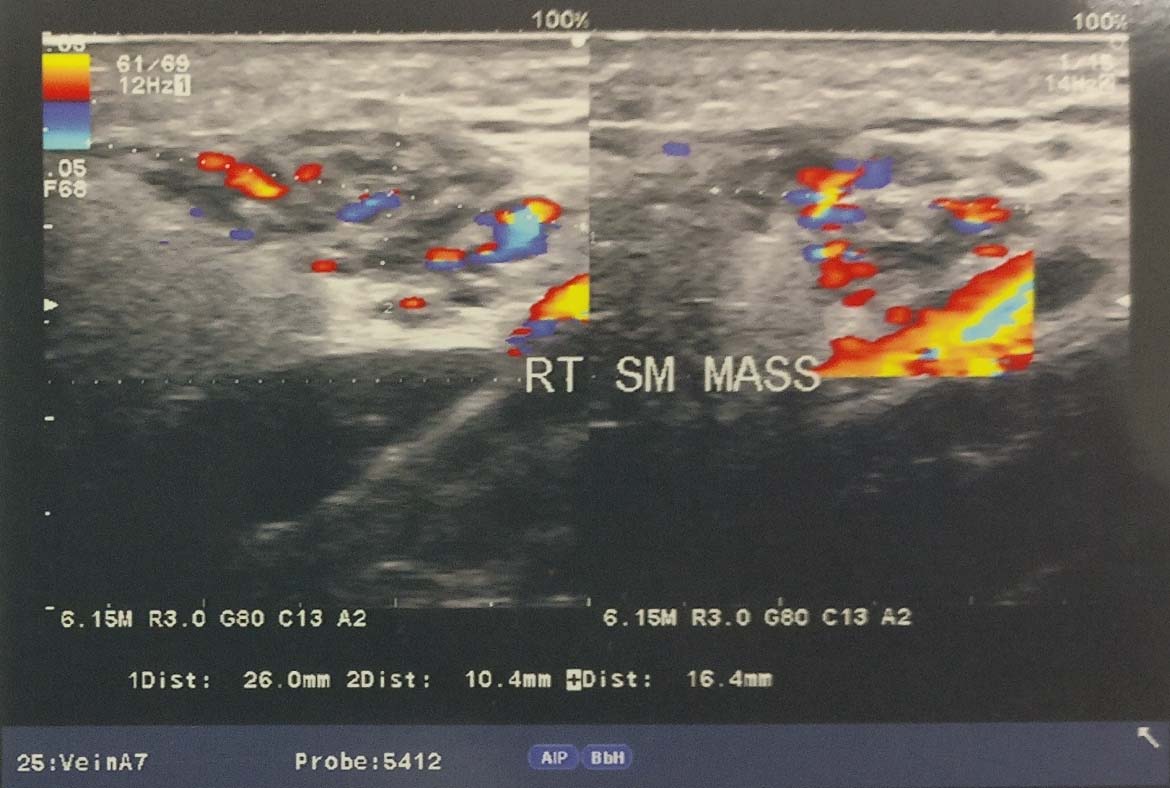
USG with Doppler study showed a mixed echoic lesion over right side neck, 26x10x16 mm located superficially and extending superiorly over parotid gland. On Doppler study, the lesion appeared moderately vascular showing multiple tortuous arterial and venous channels. Arterial channels were also prominent and showed slightly high velocity and increased diastolic flow [Table/Fig-2]. On the basis of above mentioned USG findings, a diagnosis favouring arterio-venous malformation was reported by the radiologist and MRI along with MRA was planned next to get a more clear and detailed radiological information.
USG with Doppler study neck showing a superficially located mixed echoic lesion over right side neck and extending superiorly over parotid gland. The lesion appeared moderately vascular showing multiple tortuous arterial and venous channels on Doppler.
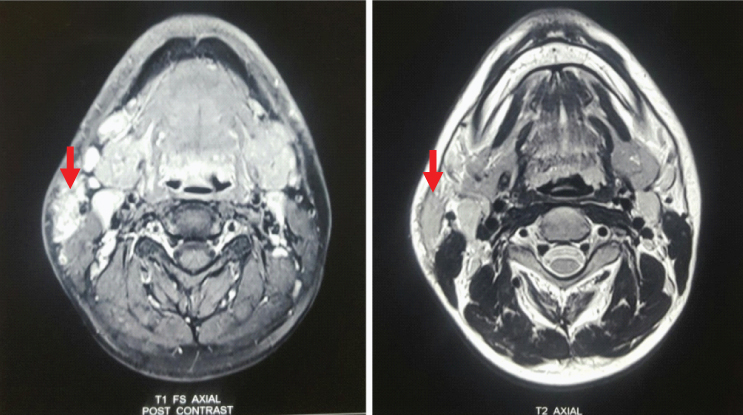
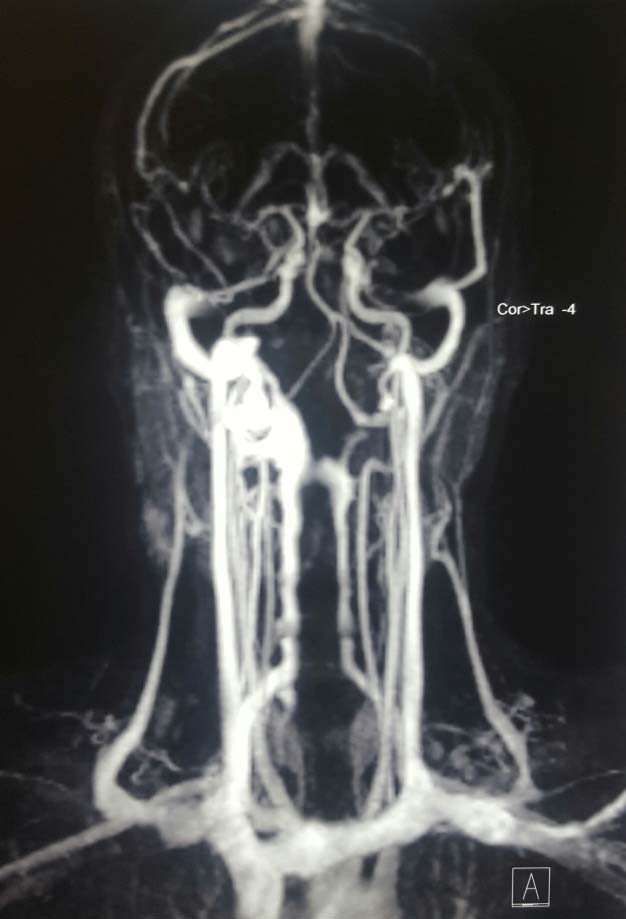
MRI revealed a soft tissue lesion in the right side of neck inferior to the superficial lobe of right parotid gland, measuring approximately 3.0x1.4x2.8 cm in maximum dimension. It was seen anterior to the sternoclenoid muscle abutting its anterior margin. The lesion was seen in close relation with the inferior pole of parotid with indistinct fat planes. It appeared hyperintense on T2 weighted images; hypointense on T1 weighted images [Table/Fig-3]. On post contrast dynamic angiogram, the lesion did not show enhancement in the arterial phase images/prominent arterial feeders. It showed progressive enhancement in delayed phase images with homogeneous enhancement. The lesion was draining into the external jugular vein [Table/Fig-4]. Mildly enlarged lymph nodes were seen in submandibular region, along the jugular chain bilaterally and submental region, largest measuring approximately 2.2x1.0 cm in size. The above MRI and MRA findings were consistent with venous malformation.
MRI with T1 and T2 weighted image in axial view showing a right subcutaneous mass (marked by bold red arrows).
MRA showing the lesion draining into external jugular vein
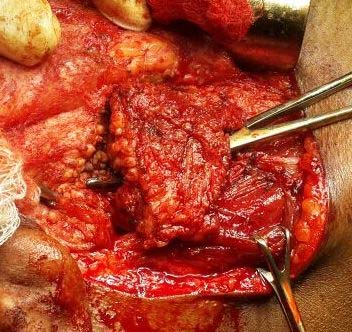
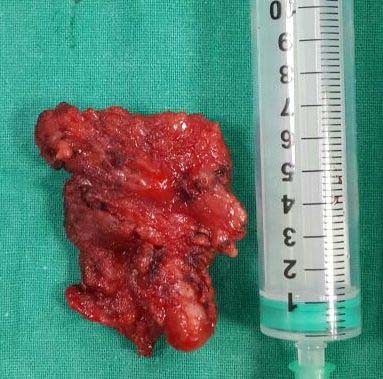
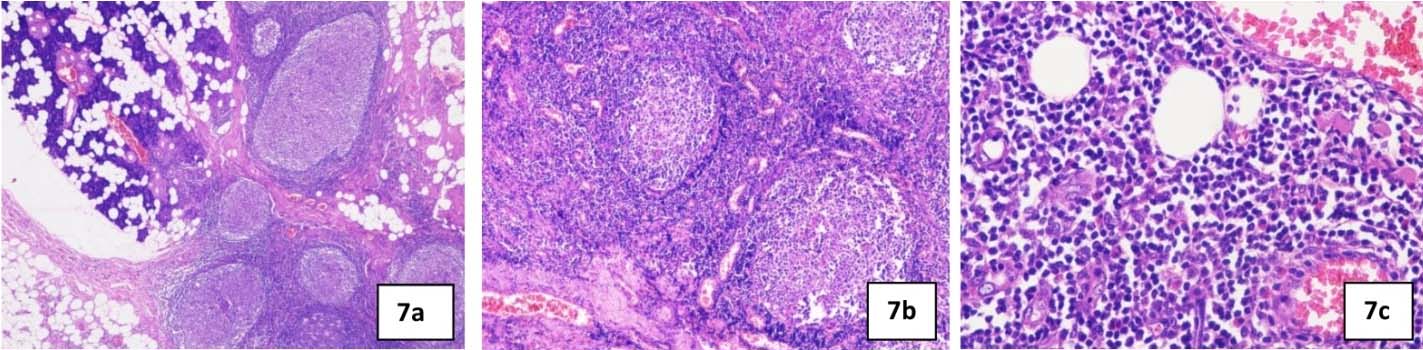
Surgical excision of the right subcutaneous mass and superficial parotidectomy was performed under general anaesthesia [Table/Fig-5]. The specimen measured approximately 7 cm in largest diameter, reddish brown in color, soft to firm in consistency, rough nodular with irregular borders [Table/Fig-6]. Postoperative recovery was good with no signs of facial nerve injury. Histological examination of surgical specimen with haematoxylin and eosin staining revealed salivary gland tissue showing lymphoid hyperplasia with germinal center formation [Table/Fig-7a], increase in number of blood vessels in the interfollicular area, some of which showed hyalinization [Table/Fig-7b] and eosinophilic infiltrate [Table/Fig-7c]. These histological features were consistent with Kimura’s disease description.
Intraoperative photograph of the lesion being excised.
Excised surgical specimen.
a) photomicrograph showing salivary gland tissue with lymphoid hyperplasia and germinal center formation (H&E 4x); 7b) photomicrograph showing increase in number of blood vessels in the interfollicular area, some of which shows hyalinization (H&E 10x); 7c) photomicrograph showing eosinophilic infiltrate (H&E 40x).
The patient has been followed up in outpatient department and has been asymptomatic for the last one year without being on any medical management.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review.
Discussion
KD is a chronic disease with benign nature, and is rarely seen. KD is seen most commonly in the head and neck region, the presentation is a deep subcutaneous mass with associated regional lymphadenopathy or salivary gland involvement. Peripheral eosinophilia and elevated IgE levels in the laboratory findings are
most attributed to diagnosis of KD. To the best of our knowledge, only three cases of KD with normal serum eosinophil count or/and IgE levels have been reported worldwide and none reported from Indian subcontinent [1-3]. To the best of our knowledge, our’s is the first reported case of KD from Indian subcontinent with normal serum eosinophil count and IgE levels. Additionally, KD mimicking venous malformation on imaging studies have been reported only once from Indian population [4], with our case being second.
KD was first described in China in 1937 and was later characterized by Kimura T et al., [5,6]. It is synonymous with eosinophilic granuloma, eosinophilic hyperplasia lymphogranuloma, eosinophilic lymphofollicular granuloma [7,8].
Although the disease can manifest at any age, most cases have been reported in second and third decades of life. The mean age being 31 years in a case series of patients from US [3]. In other case series of 54 Chinese patients, the mean age of the patients was 33.1 years [9]. The youngest age reported has been 15 months [10]. There is a male predominance, with six times as many males being affected than females [3]. Although it shown to have no racial predisposition in a case series of patients from US [3], majority of patients with KD have been reported from Asian populations in Japan [11], China [9,12], Taiwan [13] and Hong Kong [14]. Sporadic cases have been reported from other ethnic groups [15-18]. Uncommon in Indian natives, with only 20 cases reported from Indian population until 2013 [19]. Majority of cases reported in the literature occurred in the head and neck (76%) [3], in addition to buttock, axilla, groin, arm and femoral region [2,5,20-22].
No clear conclusions have been made about the exact cause and pathogenesis of KD; however various theories have been postulated. Although allergic, autoimmune, infective and neoplastic causes were proposed, no infective agent or allergen has been isolated so far in lesions of Kimura’s disease [23]. Arthropod bites, Ebstein-Barr virus, Human herpes virus-8, Candida albicans and parasitic infection have been suggested [24-26]. KD in a haemodialysis patient has been reported once [27]. Immune reaction is thought to be the root of Kimura’s disease and is supported by the presence of peripheral eosinophils, increased mast cells and increased levels of interleukin (IL)-5 and IgE, which imply an abnormal T cell stimulation [3,28].
A total of 60% of cases of KD have associated renal involvement [29]. In those cases, the renal pathologies showed almost all types of glomerulonephritis [30,31]. In addition, association between KD and asthma, Raynaud phenomenon, Lichen amyloidosus, Ulcerative colitis, Temporal arteritis, erythroderma, eosinophilic myocarditis and eosinophilic panniculitis have also been reported [32-37].
There is not fixed set of signs and symptoms associated with KD. Patients may present with a single or multiple subcutaneous nodules. These nodules are frequently pruritic and may also be painful in some cases. These nodules are slow growing. The diseases mimicking the findings of KD and may be confused with KD are Angiolymphoid Hyperplasia with Eosinophilia (ALHE), vascular malformations [38], pharyngeal pouch, tuberculous abscess, Castleman’s disease, Hodgkin’s lymphoma, Mikulicz’s disease [39], nodal metastasis and reactive lymphadenopathy.
ALHE is most commonly confused with KD. These two disorders are sometimes known to occur in the same patient [40]. ALHE and KD were initially considered same entities by few authors. Rosai J et al., in 1979 eventually clarified this misconception, and KD and ALHE were established as two distinct entities [41]. The two diseases are differentiated on the basis of clinical, laboratory and histological findings [Table/Fig-8] [2,24].
Comparison between Kimura’s disease and ALHE.
| Kimura’s disease | ALHE |
|---|
| Age | 2nd-4th decate | 2rd-5th decate |
| ses | M>F | F>M |
| Demography | Japan, china, Korea | Any, mostly Caucasian |
| Location | Head & neck mostly | Various locations |
| Lymphadenopathy | Common | Uncommon |
| Peripheral eosinophilia | Usually present | Prsent in <10% |
| Serum lgE | Usually elevated | Usually normal |
| Serum ESR | Usually raised | Usually normal |
| Histology | Thin walled blood vessels with cubical endothelial lining | Hypertrophied and sometimes vacuolated endothelial cells with eosinophilic cytoplasm |
| Immunofluorescence | Heavy lgE deposit in germinal centers | Germinal centers lacks lgE deposit |
| Nephrepathy | Associated in 29-50% patients | No association |
Diagnosis of KD can be difficult and misleading. Most consistent laboratory findings are elevated serum IgE level and peripheral eosinophilia. Absence of above laboratory findings, as in our case can lead to diagnostic dilemma. Only few cases of KD; three to our best knowledge have been reported with normal peripheral eosinophil and/or serum IgE levels. Yang T-H et al., reported KD of left side of neck with normal eosinophil and serum IgE levels [1]. Choi WJ et al., reported a KD occurring on the buttock of a five-year-old boy with normal peripheral eosinophil count while elevated serum IgE level (1,698 ng/ml) [2]. In a case series of patients from US [3], normal peripheral eosinophil level was documented in one out of 17 patients tested, while normal serum IgE level was detected in one out of nine patients tested. One study has shown a positive correlation between degree of blood eosinophilia and size of lesion and hence disease activity [42]. KD with high IgG-4 has also been reported [43]. Impaired renal function tests, leukocytosis, elevated ESR and C-reactive proteins are other possible laboratory findings.
Imaging studies can help in staging the extent and progression of disease, lymph node and parotid gland involvement and to rule
out other causes of neck masses. There is no strong consensus on characteristic imaging findings suggestive of KD. Park SW et al., reported specific intensity and signal changes in CT and MRI examination in lesions of KD [44] while Zhang R et al., showed no specific signal and intensity changes [45]. On radiological examination, KD mimics other chronic and neoplastic diseases. Yang T-H et al., reported a case of KD simulating Hodgkin’s lymphoma on 18F-fluorodeoxyglucose positron emission tomography and computed tomography (18F FDG PET-CT) [1]. Rodrigues G et al., reported a doubtful pulsatile thigh swelling which was diffusely vascular on MR angiogram mimicking vascular malformation as in our case [4]. Therefore, diagnosis of KD cannot be based exclusively on imaging studies.
FNA is mostly inconclusive, or may yield a false diagnosis of reactive lymphadenitis [27] or chronic sialadenitis [46]. Both KD and ALHE cannot be differentiated cytologically [47].
Definitive diagnosis of KD is based on histological findings. Repeated biopsy might be required in uncertain cases if the histological findings are not typical for KD [48,49]. In 1989, Hui PK et al., gave histological classification features of KD as constant, frequent and rare [14]. Hyperplasia of germinal centers, intact nodal morphology, post-capillary venule proliferation and eosinophilic infiltrate are most constant histological features. Frequent findings include multinucleated giant cells, sclerosis, eosinophilic microabcessses, necrosis and vascularization of the germinal centers and florid proteinaceous material deposits in the germinal centers. The solitary rare feature is the progressive transformation of the germinal centers. Immunochemistry reveals the presence of IgE reticular network in germinal centers and IgE coated non-degranulated mast cells.
There is no gold standard treatment option and well defined treatment protocol for KD is yet to be established. However, treatment should aim to preserve function and aesthetics while preventing recurrences and long term sequels [50]. In asymptomatic cases, conservative observation is often adequate as lesions occasionally undergo spontaneous resolution. Surgical excision is considered as first line of treatment in symptomatic cases [28]; although recurrence (25%) is common [51]. Topical and systemic steroids are frequently used to prevent frequent relapse and in cases with concomitant nephrotic syndrome, but tumors may become refractory to treatment. Radiation therapy is useful to control lesions that are not responsive to steroids or with a relapse after surgery. Effective total dose is proved to be 20-30 Gy [52]. Recently, use of imatinib, cyclosporine, azathioprine, pentoxifylline and pranlukast has even been validated [35,50,53]. Antihistaminics like cetrizine [53] has been tried prior to corticosteroids for sparing the effects of systemic steroid in this disease. Successful use of photodynamic therapy in recurrence of KD after surgery has been reported [54].
The overall prognosis is good with no malignant transformation documented till date [13]; although, recurrences after all form of treatment are present. The recurrence of disease is affected by various factors, including disease duration, lesion diameter, blood eosinophil count, well-defined lesion boundaries, serum IgE levels and single or multiple lesions [55].
Abbreviations
KD: Kimura’s disease
ALHE: Angiolymphoid hyperplasia with eosinophilia
IgE: Immunoglobulin E
USG: Ultrasonography
MRI: Magnetic resonance imaging
MRA: Magnetic resonance angiogram
CT: Computed tomography
18F FDG PET-CT: 18F-fluorodeoxyglucose positron emission tomography and computed tomography
FNA: Fine needle aspiration
IL: Interleukin
ESR: Erythrocyte sedimentation rate
IgG-4: Immunoglobulin G-4
Gy: Gray
Conclusion
KD is rare condition mostly seen in orientals and only few sporadic cases reported from Indian subcontinent. It can simulate venous malformation on imaging studies. Additionally, absence of peripheral eosinophilia can lead to a diagnostic dilemma. Histological diagnosis is only confirmatory.
[1]. Yang T-H, Chou Y-H, Kao W-Y, Cherng S-C, Kimura disease simulating hodgkin’s lymphoma on 18F FDG PET-CT:report of a caseNuclear Medicine and Molecular Imaging 2014 48(4):313-16. [Google Scholar]
[2]. Choi WJ, Hur J, Ko JY, Yeo KY, Kim JS, Yu HJ, An unusual clinical presentation of kimura’s disease occurring on the buttock of a five-year-old boyAnnals of Dermatology 2010 22(1):57-60. [Google Scholar]
[3]. Chen H, Thompson LD, Aguilera NS, Abbondanzo SL, Kimura disease:a clinicopathologic study of 21 casesAm J SurgPathol 2004 28:505-13. [Google Scholar]
[4]. Rodrigues G, Ravi B, Synchronous Kimura lesions at two different sites–a diagnostic dilemma! Quantitative Imaging in Medicine and Surgery 2016 6(2):214-17. [Google Scholar]
[5]. Kimm HT, Szeto C, Eosinophilic hyperplastic lymphogranuloma, comparison with Mikulicz’s diseaseChin Med J 1937 23:699-700. [Google Scholar]
[6]. Kimura T, Yoshimura S, Ishikawa E, On the unusual granulation combined with hyperplastic changes of lymphatic tissueTrans Soc Pathol Jpn 1948 37:179-80. [Google Scholar]
[7]. Helander SD, Peters MS, Kuo TT, Su WP, Kimura’s disease and angiolymphoid hyperplasia with eosinophilia:new observations from immunohistochemical studies of lymphocyte markers, endothelial antigens, and granulocyte proteinsJ Cutan Pathol 1995 22:319-26. [Google Scholar]
[8]. Mitsui M, Ogino S, Ochi K, Ohashi T, Three cases of eosinophilic lymphfolliculoid granuloma of the soft tissue originating from the parotid glandActa Otolaryngol Suppl 1996 522:130-32. [Google Scholar]
[9]. Li TJ, Chen XM, Wang SZ, Fan MW, Semba I, Kitano M, Kimura’s disease:a clinicopathologic study of 54 Chinese patientsOral Surg Oral Med Oral Pathol Oral Radiol Endod 1996 82:549-55. [Google Scholar]
[10]. Dixit MP, Scott KM, Bracamonte E, Dixit NM, Schumacher MJ, Hutter J, Kimura disease with advanced renal damage with anti-tubular basement membrane antibodyPediatr Nephrol 2004 19:1404-07. [Google Scholar]
[11]. Urabe A, Tsuneyoshi M, Enjoji M, Epithelioid haemangioma versus Kimura’s disease:a comparative clinicopathologic studyAm J Surg Pathol 1987 11:758-66. [Google Scholar]
[12]. Zhang JZ, Zhang CG, Chen JM, Thirty-five cases of Kimura’s disease (eosinophilic lymphogranuloma)Br J Dermatol 1998 139:542-43. [Google Scholar]
[13]. Kuo TT, Shih LY, Chan HL, Kimura’s disease:involvement of regional lymph nodes and distinction from angiolymphoid hyperplasia with eosinophiliaAm J Surg Pathol 1988 12:843-54. [Google Scholar]
[14]. Hui PK, Chan JK, Ng CS, Kung ITM, Gwi E, Lymphadenopathy of Kimura’s diseaseAm J Surg Pathol 1989 13:177-86. [Google Scholar]
[15]. Chusid MJ, Rock AL, Sty JR, Oechlerc HW, Beste DJ, Kimura’s disease:an unusual cause of cervical tumourArch Dis Child 1997 77:153-54. [Google Scholar]
[16]. Hamrick HJ, Jennette JC, La Force CF, Kimura’s disease:report of a pediatric case in the United StatesJ Allergy Clin Immunol 1984 73:561-66. [Google Scholar]
[17]. Jaber K, Kimura’s disease in an Arab femaleHistopathology 1996 29:76-78. [Google Scholar]
[18]. Pamaraju N, Khalifa SA, Darwish A, Paulose KO, Ahmed N, Yousif H, Kimura’s diseaseJ Laryngol Otol 1996 110:1084-87. [Google Scholar]
[19]. Sah P, Kamath A, Aramanadka C, Radhakrishnan R, Kimura’s disease - An unusual presentation involving subcutaneous tissue, parotid gland and lymph nodeJ Oral Maxillofac Pathol 2013 17:455-59. [Google Scholar]
[20]. Prasad BK, Deviprasad R, Kimura’s disease:An unusual case of neck massIndian J Otolaryngol Head Neck Surg 2008 60:353-55. [Google Scholar]
[21]. Song KJ, Lee KB, Kimura’s disease occurred in the whole armJoint Bone Spine 2008 75(1):76-77. [Google Scholar]
[22]. Varshney MK, Kumar A, Khan SA, Yadav CS, Kimura disease of extremity:unusual manifestation in a long boneJoint Bone Spine 2008 75(4):492-94. [Google Scholar]
[23]. Chong WS, Thomas A, Goh CL, Kimura’s disease and angiolymphoid hyperplasia with eosinophilia:Two disease entities in the same patient:Case report and review of the literatureInt J Dermatol 2006 45:139-45. [Google Scholar]
[24]. Dik VK, van der Wiel BA, Vasmel WL, Kimura’s disease of the parotid glands and multiple cervical lymph nodesNeth J Med 2010 68:175-77. [Google Scholar]
[25]. Armstrong WB, Allison G, Pena F, Kim JK, Kimura’s disease:Two case reports and a literature reviewAnn Otol Rhinol Laryngol 1998 107:1066-71. [Google Scholar]
[26]. Nagore E, Llorca J, Sánchez-Motilla JM, Ledesma E, Fortea JM, Aliaga A, Detection of Epstein-Barr virus DNA in a patient with Kimura’s diseaseInt J Dermatol 2000 39(8):618-20. [Google Scholar]
[27]. Lee S, Jung SJ, Park SK, Kang KP, Jang KY, Kang MJ, Kimura’s Disease Involving Thoracic and Abdominal Lymph Nodes in a Haemodialysis PatientThe Korean Journal of Internal Medicine 2005 20(2):159-62. [Google Scholar]
[28]. Meningaud JP, Pitak Arnnop P, Fouret P, Bertrand JC, Kimura’s disease of the parotid region:Report of 2 cases and review of the literatureJ Oral Maxillofac Surg 2007 65:134-40. [Google Scholar]
[29]. Yuen HW, Goh YH, Low WK, Lim-Tan SK, Kimura’s disease:a diagnostic and therapeutic challengeSingapore Med J 2005 46:179-83. [Google Scholar]
[30]. Ranka SR, Rajput A, Kantharia CV, Kimura’s diseaseIndian Journal of Otolaryngology and Head &Neck Surgery 2004 56(1):43-45. [Google Scholar]
[31]. Rajpoot DK1, Pahl M, Clark J, Nephrotic syndrome associated with Kimura diseasePediatr Nephrol 2000 14(6):486-88. [Google Scholar]
[32]. Barber J, Dawes P, A rare cause of rash, eosinophilia and asthma in rheumatologyRheumatology 2002 41:1329 [Google Scholar]
[33]. Teraki Y, Katsuta M, Shiohara T, Lichen amyloidosus associated with Kimura’s disease:successful treatment with cyclosporineDermatology 2002 204(2):133-35. [Google Scholar]
[34]. Shimamoto C, Takao Y, Hirata I, Ohshiba S, Kimura’s disease (angiolymphoid hyperplasia with eosinophilia) associated with ulcerative colitisJournal of Gastroenterology 1993 28(2):298-303. [Google Scholar]
[35]. Sun QF, Xu DZ, Pan SH, Ding JG, Xue ZQ, Miao CS, Kimura disease:review of the literatureIntern Med J 2008 38(8):668-72. [Google Scholar]
[36]. Nakagawa C, Sakaguchi Y, Nakajima T, Kawamoto A, Uemura S, Fujimoto S, A case of eosinophilic myocarditis complicated by Kimura’s disease (eosinophilic hyperplastic lymphogranuloma) and erythrodermaJpn Circ J 1999 63(2):141-44. [Google Scholar]
[37]. Maleki D, Sayyah A, Rahimi-Rad MH, Gholami N, Kimura’s disease with eosinophilicpanniculitis - treated with cyclosporine:a case reportAllergy, Asthma, and Clinical Immunology: Official Journal of the Canadian Society of Allergy and Clinical Immunology 2010 6(1):5 [Google Scholar]
[38]. Bharati V H, Kalaivani V, Bharathi R, Vineet B, Kimura’s Disease – A Diagnostic And Therapeutic DilemmaJournal of Clinical and Diagnostic Research 2012 6(2):311-12. [Google Scholar]
[39]. Guimaraes CS, Moulton-Levy N, Sapadin A, Vidal C, Kimura’s DiseaseCase Reports in Medicine 2009 2009:424053 [Google Scholar]
[40]. Reddy PKS, Prasad ALS, Sumathy TK, Shivaswamy KN, Ranganathan C, An overlap of angiolymphoid hyperplasia with eosinophilia and Kimura’s disease:successful treatment of skin lesions with cryotherapyIndian Journal of Dermatology 2015 60(2):216 [Google Scholar]
[41]. Rosai J, Gold J, Landy R, The histiocytoid haemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heartHum Pathol 1979 10:707-30. [Google Scholar]
[42]. Sakamoto M, Komura A, Nishimura S, Haematoserological analysis of Kimura’s disease for optimal treatmentOtolaryngol Head Neck Surg 2005 132:159-60. [Google Scholar]
[43]. Li J, Ge X, Ma J, Li M, Li J, Kimura’s disease of the lacrimal gland mimicking IgG4-related orbital diseaseBMC Ophthalmology 2014 14:158 [Google Scholar]
[44]. Park SW, Kim HJ, Sung KJ, Lee JH, Park IS, Kimura disease: CT and MR imaging findingsAm J Neuroradiol 2012 33:784-88. [Google Scholar]
[45]. Zhang R, Ban XH, Mo YX, Lv MM, Duan XH, Shen J, Kimura’s disease:The CT and MRI characteristics in fifteen casesEur J Radiol 2011 80:489-97. [Google Scholar]
[46]. Tauro DP, Kumar KK, Shibani S, Hallikeri K, Unusual presentation of Kimura’s disease involving the parotid gland in an Indian male:a case report and review of literatureJournal of Oral Biology and Craniofacial Research 2012 2(1):50-52. [Google Scholar]
[47]. Tanveer N, Mishra K, Singh UR, Cytological features of Kimura’s disease:A case report with histological correlationClin Cancer Investig J 2015 4:752-55. [Google Scholar]
[48]. Savage NW, Boras VV, Unilateral intraparotid swelling:a case report of Kimura’s disease and review of differential diagnosisCase Reports in Otolaryngology 2013 2013:795921 [Google Scholar]
[49]. Iida S, Fukuda Y, Ueda T, Sakai T, Okura M, Kogo M, Kimura’s disease:report of a case with presentation in the cheek and upper eyelidJournal of Oral and Maxillofacial Surgery 2005 63(5):690-93. [Google Scholar]
[50]. Itami J, Arimizu N, Miyoshoi T, Ogata H, Miura K, Radiation therapy in Kimura’s diseaseActa Oncol.1989;28:511-14. [Google Scholar]
[51]. Larroche C, Bletry O, Kimura’s DiseaseOrphanet encyclopedia 2005 Februaryhttp://www.orpha.net/data/patho/GB/uk-kimura.pdf [Google Scholar]
[52]. Hareyama M, Oouchi A, Nagakura H, Asakura K, Saito A, Satoh M, Radiotherapy for Kimura’s disease:the optimum dosageInt J Radiat Oncol Biol Phys 1998 40(3):647-51. [Google Scholar]
[53]. Ben-Chetrit E, Amir G, Shalit M, Cetirizine:an effective agent in Kimura’s diseaseArthritis Rheum 2005 53:117-18. [Google Scholar]
[54]. Abbas S, Jerjes W, Upile T, Vincent A, Hopper C, Treatment of Kimura disease with photodynamic therapy:a case studyPhotodiagnosis Photodyn Ther 2012 9(1):83-86. [Google Scholar]
[55]. Lin YY, Jung SM, Ko SF, Toh CH, Wong AM, Chen YR, Kimura’s disease:clinical and imaging parameters for the prediction of disease recurrenceClin Imaging 2012 36(4):272-78. [Google Scholar]