Secondary Acute Angle Closure Glaucoma (AACG) is a known side effect of Topiramate (TPM). Here, we present a case report of a 47-year-old male who was started on TPM 25 mg/day for migraine. He presented to the ophthalmology department of our hospital with sudden blurring of vision and colored halos after one day of starting TPM. A high index of suspicion, followed by appropriate investigations and prompt management that helped to manage TPM induced bilateral AACG with quick and complete visual recovery.
Intraocular pressure, Methylprednisolone, Migraine
Case Report
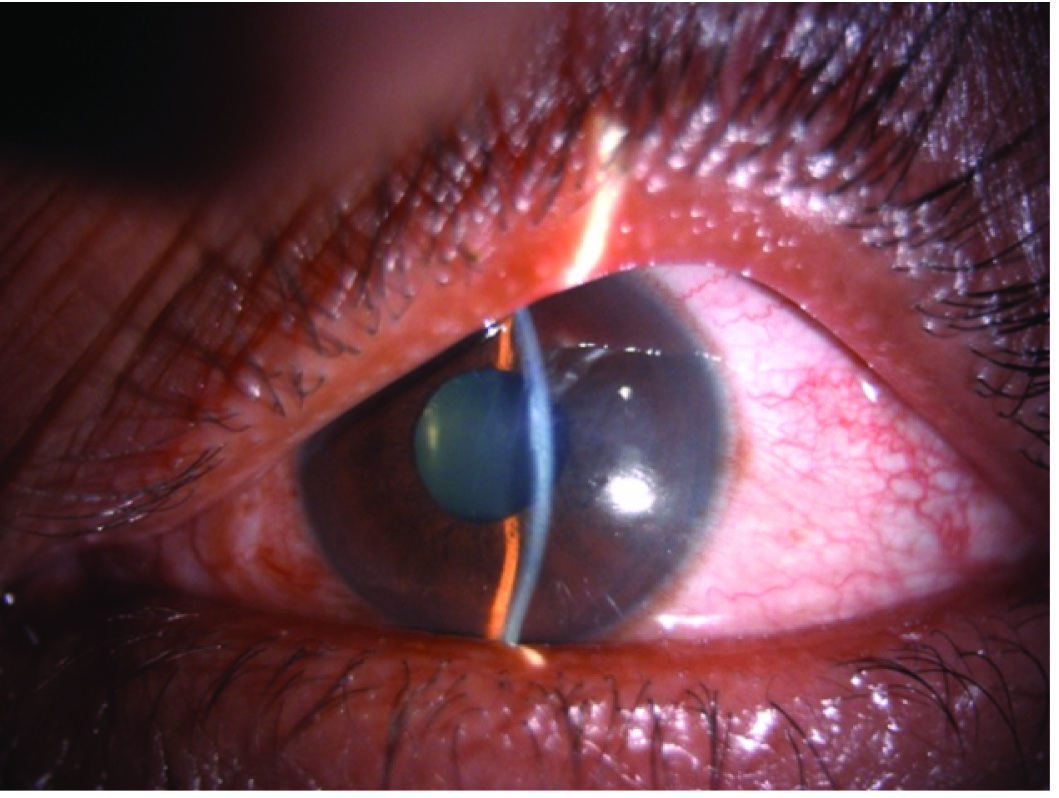
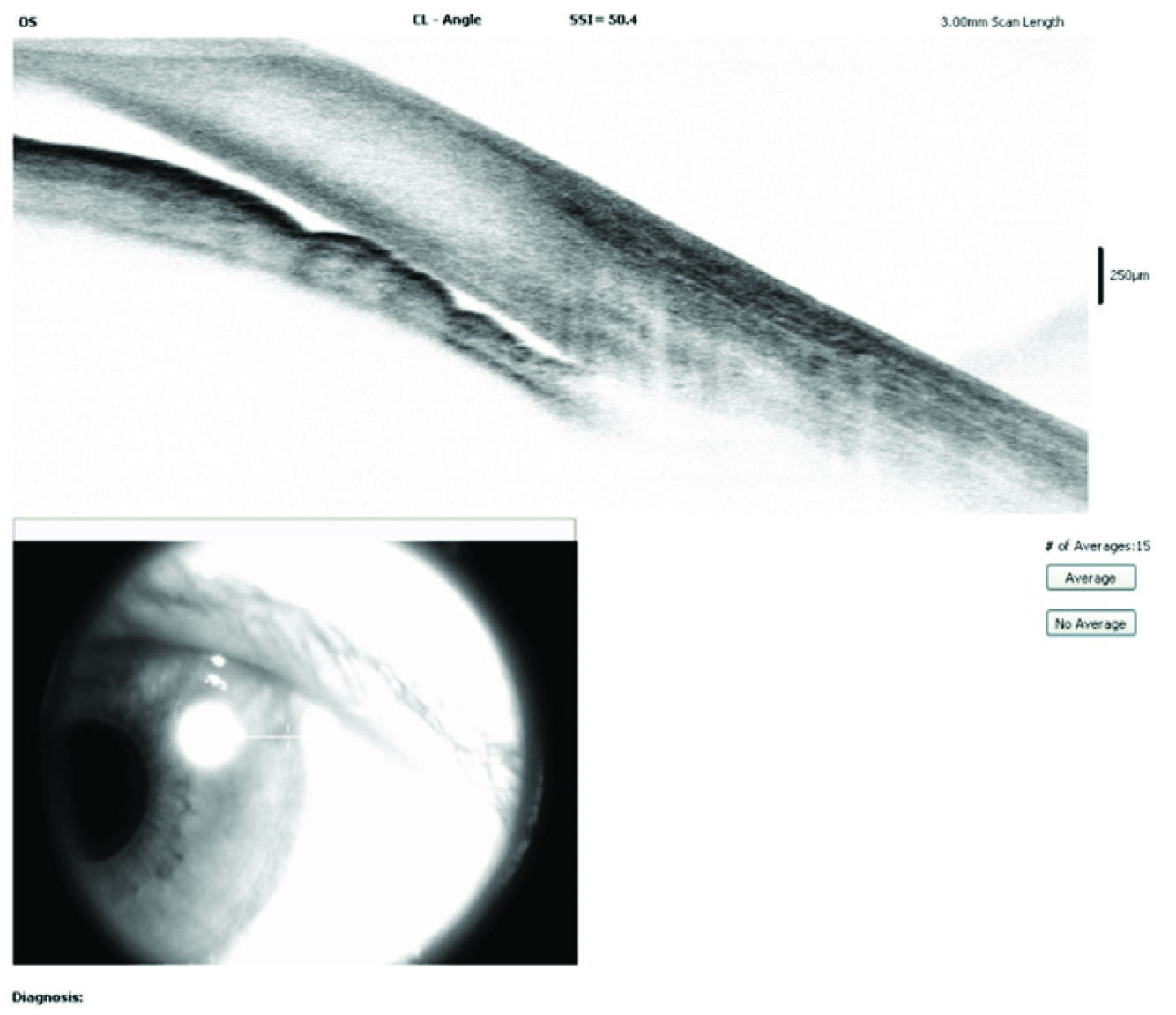
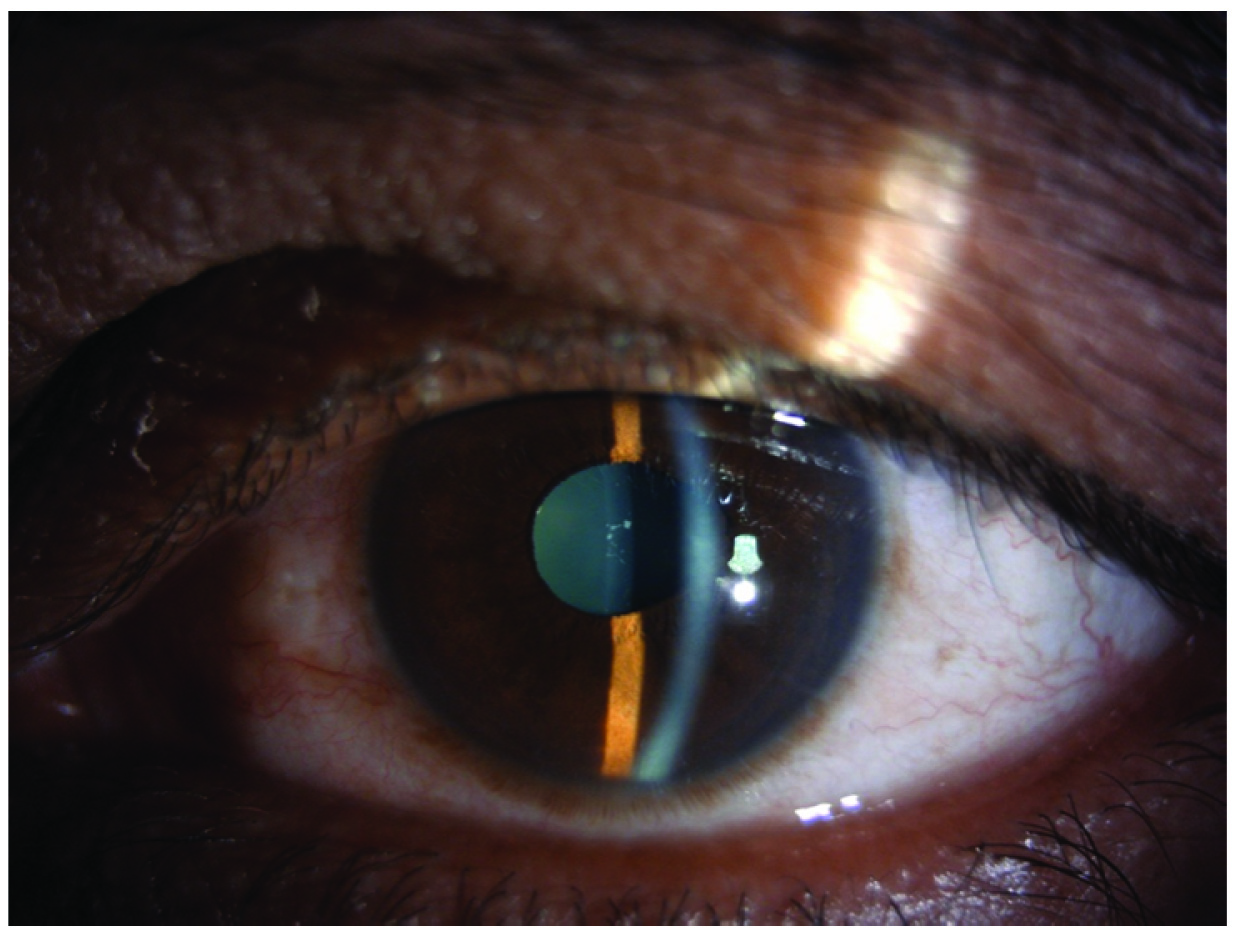
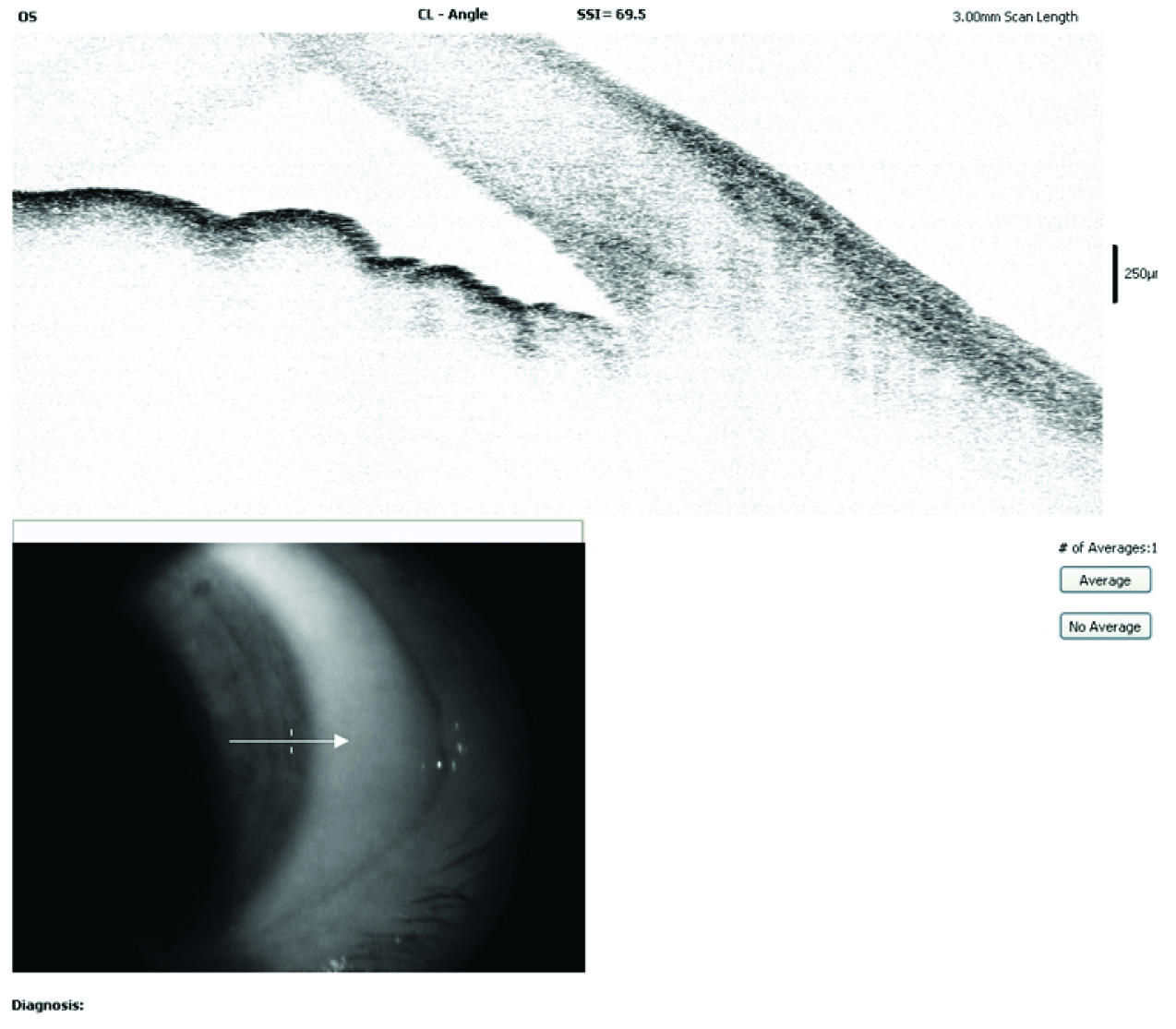
A 47-year-old male presented to the Department of Ophthalmology with sudden decrease in vision and pain in both eyes since one day. He had been recently diagnosed to have migraine and was started on tablet Topiramate (TPM) 50 mg/day for 10 days. On examination, his vision was 6/60 both eyes improving to 6/36 with -5D sphere correction. Both eyes showed ciliary congestion, corneas clear, shallow anterior chambers, clear lens and round reactive pupils [Table/Fig-1]. Intraocular Pressure (IOP) was 50 mmHg in both eyes. Gonioscopy and angle Optical Coherence Tomography (OCT) showed closed angles [Table/Fig-2] and an undilated fundus examination showed 0.4 cup with healthy rim in both eyes. Angle OCT was showing closed angles with no pupillary block and B Scan ultrasound was showing choroidal effusion/detachment. Considering the bilateral involvement and history of TPM, a diagnosis of TPM induced AACG was made. Patient was given I.V methylprednisolone 1 gm loading dose, I.V mannitol, timolol eye drops, brimonidine eye drops and TPM was stopped. Atropine eye ointment 1% was also prescribed three times a day. Patient started improving from second day with reduced intraocular pressure, decreased congestion and deep anterior chamber [Table/Fig-3]. On the third day, patient’s vision improved to 6/6 with normal IOP of 14 mmHg in both eyes and deep anterior chambers and wide open angle on gonioscopy. OCT showed normal angles [Table/Fig-4], B scan showed decrease in choroidal effusion. Over a period of one week, all medications were stopped. He was followed up after one month and was being kept on a regular six monthly follow up. Every follow up visit showed ophthalmological examination within normal limits.
Anterior segment photograph showing ciliary congestion, shallow anterior chamber in our rare case of TPM induced secondary AACG.
Anterior segment OCT showing closed angles in our rare case of TPM induced secondary AACG.
Anterior segment photograph showing decreased ciliary congestion, resolution of attack of TPM induced secondary AACG.
Anterior segment OCT showing open angles post treatment in our rare case of TPM induced secondary AACG.
Discussion
TPM is an anticonvulsant drug mainly used in the treatment of epilepsy as well as prophylactic therapy for migraine headaches [1].
It acts predominantly by inactivating the sodium or calcium gate channels, hyperpolarising K+ currents, inhibition of kainite-mediated conductance and activating GABA postsynaptic receptors. In addition, it also has some anti-carbonic anhydrase activity [2]. TPM is rapidly absorbed after oral administration and has half-life of 24 hours, being rapidly excreted in the urine. It’s possible mechanism of reduction of intracranial pressure is related to its inhibition of carbonic anhydrase activity and perhaps its induction of weight loss [2].
One rare, but potentially serious adverse event that is linked to TPM use is AACG, which may lead to blindness. Currently data on the incidence of TPM induced glaucoma is lacking [1].
In the study done by Etminan M et al., it was found that there was a slight increase in the risk of glaucoma among current users of TPM, risk was greater among new users of TPM [1].
According to Fraunfelder FW et al., study, there have been 115 case reports of ocular side effects, 86 cases of secondary angle closure glaucoma and seven cases of permanent visual loss reported with TPM, suggesting an association between acute onset of AACG after TPM therapy [3,4].
The ocular side effects of TPM include transient myopia, retinal striae, acute bilateral ACG, visual field loss, maculopathy, myopic shift, trichomegaly, vitritis, palinopsia, rhegmatogenous retinal detachment and anterior uveitis [2].
Patients on TPM present with blurred vision, headache or nausea and vomiting along with findings characteristic of an acute attack of AACG. Conventional and high frequency ultrasound demonstrated choroidal or cilio-choroidal detachments [2]. Our case had ciliochoroidal detachment.
Patients on TPM should undergo routine ophthalmological evaluation which includes visual acuity testing, slit lamp examination, tonometry and gonioscopy. Imaging techniques like ultrasound biomicroscopy, B-scan ultrasonography, Anterior segment OCT and Pentacam Scheimpflug imaging system help in diagnosing ocular side effects of TPM [5]. In our case, patient underwent comprehensive ophthalmological evaluation along with OCT of anterior segment, which showed shallow anterior chambers with ciliochoroidal effusion in both eyes.
In our case, patient was receiving tablet TPM 25 mg once a day. In 65 different observational studies describing visual side effects of TPM in 84 consumers, the dosage of TPM induced AACG ranged from 25-100 mg/day [5].
In our case, patient developed AACG in one day after starting TPM. In a case series including 86 cases of TPM induced AACG, the time of onset ranged from 1 to 49 days with a mean of seven days following the initiation of TPM therapy. It was observed that 85% of cases occurred in the first two weeks [6].
Our case responded well to cycloplegics as deepening of anterior chambers was observed after starting cycloplegics. Van Issum C et al., suggested that in TPM induced AACG, cycloplegics are effective in reducing IOP as they retract the ciliary processes. In the same study, laser PI was performed in a few patients without proving any usefulness as the chief mechanism of TPM induced AACG is not pupillary block [7]. In our case, patient improved without undergoing laser PI.
In another study by Aminlari A et al., authors were unable to differentiate primary AACG from TPM induced AACG and role of laser PI was confined to rule out primary AACG [8].
TPM should be discontinued and an alternative should be prescribed in discussion with the primary physician [2]. The treatment should include ocular hypotensive drugs, cycloplegics, hyperosmotic agents and topical steroids. Rapid resolution of an attack has been reported with the use of intravenous methylprednisolone and mannitol [2]. In our case, patient received loading dose of 1 gm methylprednisolone intravenously along with ocular hypotensives, topical steroids and cycloplegics. The patient improved dramatically in two days of initiating treatment.
A case of TPM induced secondary AACG needs to be differentiated from primary AACG as their clinical presentation, mechanism of action, investigations and treatment differ. The differences have been summarized in [Table/Fig-5].
Comparative analysis of primary AACG with TPM induced secondary AACG.
| Categories | Primary AACG | TPM induced secondary AACG |
|---|
| Age group | Middle aged individuals | All age groups |
| Sex | Females affected more | No sex predilection |
| Family history of glaucoma | Yes | No |
| Systemic conditions associated | Not significant | Migraine, bipolar disorder, depression |
| Mechanism of angle closure | Involves pupillary block | Does not involve pupillary block. Forward shift of lens iris diaphragm |
| Gonioscopy | Angle occluded | Angle occluded |
| SLE | Corneal edema, shallow anterior chamber | Corneal edema, shallow anterior chamber |
| Bilaterality | Initially unilateral, other eye may get involved later | Near simultaneous bilateral presentation |
| B scan findings | No significant findings | Uveal effsusion, ciliary body edema |
| Role of PI | Effective | Not effective |
| Role of pilocarpine | Effective | Not effective |
| Role of cycloplegics | Contraindicated | Effective |
| Role of steroids | Topical steroids can be given | Topical and system steroids are must |
Conclusion
Misdiagnosing TPM induced AACG as primary AACG is a common diagnostic mistake and must be avoided as their treatments differ. A high index of suspicion followed by appropriate investigations and prompt management will help in managing TPM induced bilateral acute angle closures with quick and complete visual recovery.
With the increasing use of TPM especially in younger adults, potentially serious adverse idiosyncratic reactions like secondary AACG should be kept in the minds of practicing physicians. Hence, before prescribing TPM physicians should warn and advise the patient to report any cases of visual disturbances immediately.
[1]. Etminan M, Maberley D, Mikelberg FS, Use of topiramate and risk of glaucoma: a case-control studyAm J Ophthalmol 2012 153(5):827-30. [Google Scholar]
[2]. Ocular side effects of Topiramate- Frequently asked questionsThe Royal College of Ophthalmologists 2010 October [Google Scholar]
[3]. Fraunfelder FW, Fraunfelder FT, Keats EU, Topiramate-associated acute, bilateral, secondary angle-closure glaucomaOphthalmology 2004 111:109-11. [Google Scholar]
[4]. Fraunfelder FW, Fraunfelder FT, Adverse ocular drug reactions recently identified by the National Registry of Drug Induced Ocular Side EffectsOphthalmology 2004 111:1275-79. [Google Scholar]
[5]. Abtahi MA, Abtahi SH, Fazel F, Roomizadeh P, Etemadifar M, Jenab K, Topiramate and the vision: a systematic reviewClinical Ophthalmology (Auckland, N.Z.) 2012 6:117-31. [Google Scholar]
[6]. Ho JD, Keller JJ, Tsai CY, Liou SW, Chang CJ, Lin HC, Topiramate use and the risk of glaucoma development: A population-based follow-up studyAmerican Journal of Ophthalmology 2013 155(2):336-41. [Google Scholar]
[7]. Van Issum C, Mavrakanas N, Schutz JS, Shaarawy T, Topiramate-induced acute bilateral angle closure and myopia: pathophysiology and treatment controversiesEur J Ophthalmol 2011 21(4):404-09. [Google Scholar]
[8]. Aminlari A, East M, Wei W, Quillen D, Topiramate induced acute angle closure glaucomaOpen Ophthalmol J 2008 2:46-47. [Google Scholar]